
Atoms, elements, and compounds (GCSE)
KS4 National Curriculum Statement(s) covered
- A simple model of the atom consisting of the nucleus and electrons, relative atomic mass, electronic charge, and isotopes
- Position of elements in the Periodic Table in relation to their atomic structure and arrangement of outer electrons
Skip to:
Atoms are the building blocks of matter. Everything around us is made up of atoms, from the air we breathe to the ground we walk on.
Elements
An element is a pure substance made of only one type of atom. The Periodic Table arranges elements in order of increasing atomic number. The position of an element in the Periodic Table gives information about its atomic structure and properties.
Each element has a unique symbol, usually derived from its English or Latin name. Here are a few examples:
- Hydrogen (H)
- Helium (He)
- Carbon (C)
- Sodium (Na) (from Latin "Natrium")
The use of symbols for elements provides a universal and concise way to represent chemical elements in scientific communication. Each element is assigned a unique one-letter or two-letter symbol, where the first letter is always upper-case, and the second letter is always lower-case.
Symbols simplify the representation of elements in chemical equations, laboratory notes, and scientific literature. Instead of writing out full names, which can be cumbersome and lead to translation errors, symbols provide a standardised shorthand that saves space and improves clarity.
The Modern Atomic Model
Atoms are often drawn as flat (2D) structures, when in reality they are spherical (3D). An atom has a diameter of about 0.0000000001 metres (0.1 nanometres), with about 99% of an atom being empty space. The radius of a nucleus is less than 1/10000th the size of the atom.
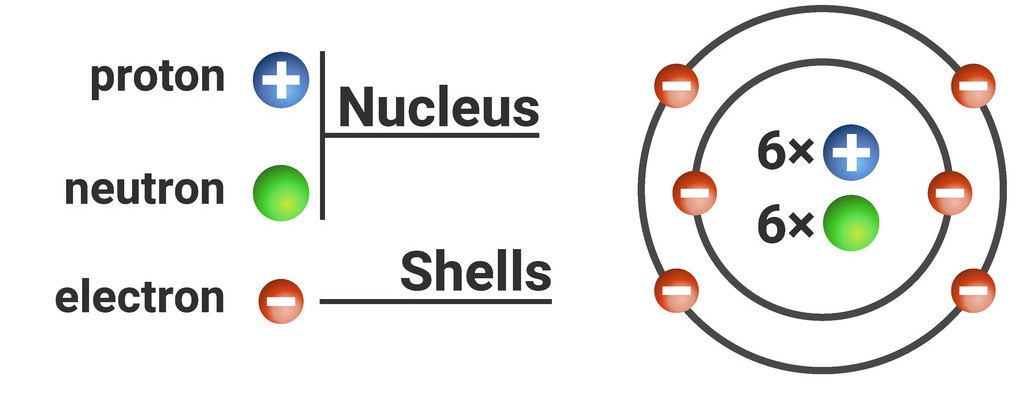
The nucleus is the dense centre of the atom. It contains two subatomic particles: protons (which have a positive charge) and neutrons (which have no charge - they are neutral). The charge on these particles is represented with a superscript number and/or symbol as shown in the table below. In chemistry, where there is only 'one' of something, you never write the number '1' - you only put the symbol and/or charge (e.g. p⁺ is really p¹⁺).
| subatomic particle | relative mass | relative charge | location in atom | symbol |
|---|---|---|---|---|
| proton | 1 | +1 | nucleus | p⁺ |
| neutron | 1 | 0 | nucleus | n⁰ |
| electron | very small/ negligible | -1 | shells | e⁻ |
The relative mass of subatomic particles is a comparison of their masses to each other. Protons and neutrons have a relative mass of 1, meaning they are approximately equal in mass. Electrons have a very small or negligible relative mass compared to protons and neutrons, making their mass effectively insignificant when calculating the total mass of an atom.
The relative charge of subatomic particles refers to the charge they carry in comparison to each other. Protons have a relative charge of +1, meaning they are positively charged. Neutrons have a relative charge of 0, indicating they are neutral and carry no charge. Electrons have a relative charge of -1, meaning they are negatively charged. The charges of protons and electrons are equal in magnitude but opposite in sign, balancing each other out in a neutral atom.
The nucleus holds the vast majority of the atom's mass, with electron mass being insignificant to the total mass of an atom.
- Protons: These positively charged particles determine the element's identity. For example, hydrogen has 1 proton, helium has 2.
- Neutrons: These neutral particles add mass to the nucleus but do not affect the atom's charge.
- Electrons: These negatively charged particles orbit the nucleus in electron shells. Electrons are much smaller than protons or neutrons and are responsible for the atom's chemical properties and reactivity.
In a neutral atom, the numbers of protons and electrons will be the same. This is because protons have a positive charge, and electrons have a negative charge. When there are equal numbers of both, they cancel out the charges. Some smaller atoms have equal numbers of protons and neutrons, however heavier atoms tend to have different numbers of both.
Electrons orbit around an atom in a certain pattern. Surrounding the nucleus are different energy levels, often called shells. Each shell can hold a specific number of electrons, and these limits are the same for every element. Each shell must be filled before electrons can occupy the next shell.
The maximum number of electrons each shell can hold is:
- 2 electrons in the first shell
- 8 electrons in the second shell
- 8 electrons in the third shell
The electron distribution throughout the shells of an atom can be represented by either writing a short code, or drawing a diagram. This is known as the electronic configuration. Crosses (×) or dots (•) are often used to represent the electrons in the diagram form. The nucleus is often left undrawn as this type of diagram is only focussing on the arrangement of electrons. It's often seen as best practice to include the element symbol in place of the nucleus.
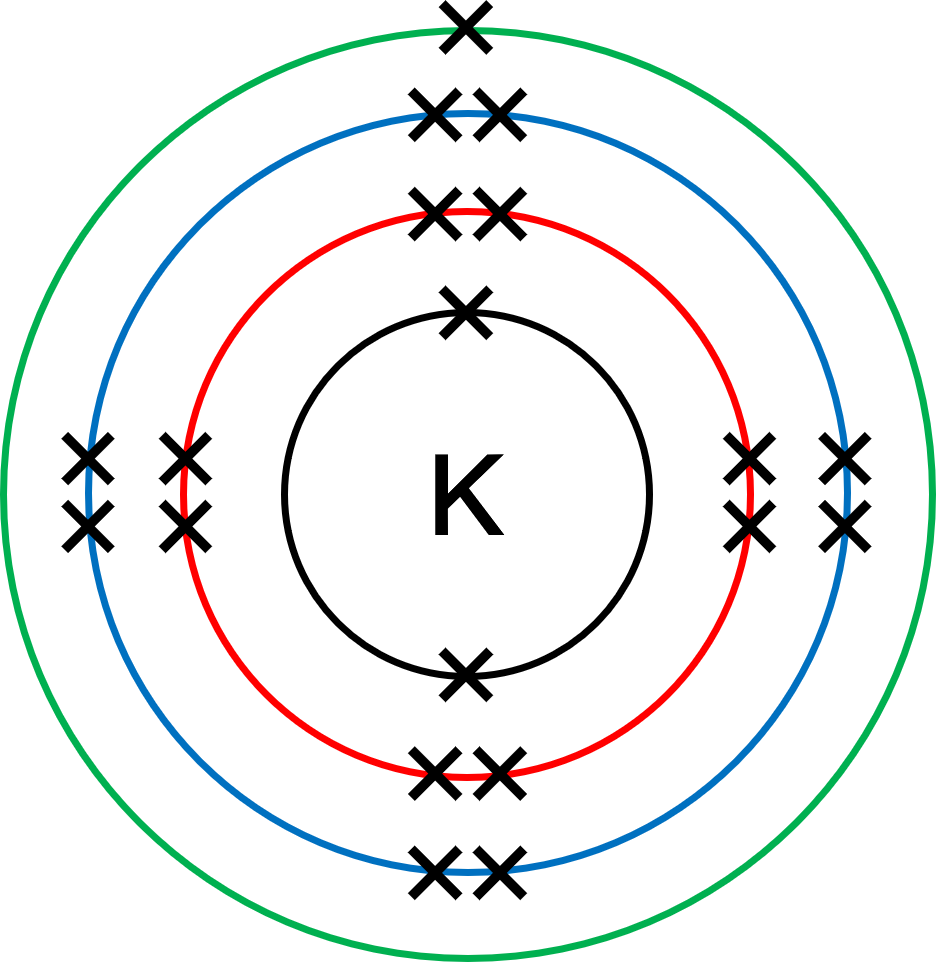
Potassium atoms have 19 electrons. The written electronic configuration of a potassium atom is therefore 2.8.8.1 because all inner shells must be filled before electrons can occupy the outer shell.
Isotopes
The number of protons in the nucleus of each atom of an element is called the atomic number. The sum of protons and neutrons is the mass number (e.g. if an atom has 6 protons and 6 neutrons, its atomic mass number is 12). The relative atomic mass (often shortened to Ar or RAM) is different to the mass number, and can refer to either:
- The relative atomic mass of a single atom, compared to 1/12th of the mass of a carbon-12 atom
- The weighted average of the masses of all isotopes of an element, based on their natural abundance

Isotopes are atoms of the same element that have different numbers of neutrons. This means they have the same atomic number but different mass numbers. Isotopes are often written with their element name (or symbol), followed by the mass number for that isotope. For example, carbon-12 (C-12) and carbon-14 (C-14) are isotopes of carbon. Both have 6 protons, but carbon-12 has 6 neutrons, while carbon-14 has 8 neutrons.
Standard nuclear notation is a way of representing the different isotopes of an element. It includes the element's symbol, the atomic number (number of protons), and the mass number (number of protons and neutrons).
General layout: AZX where A is the mass number, Z is the atomic number, and X represents the element's symbol.
For example: chlorine-35 (Cl-35)3517Cl
Worked Example - RAM calculation using percentages
A weighted average takes into account the relative proportions (abundances) of different isotopes of an element.
To find the weighted average:
- Multiply the mass of each isotope by its abundance.
- Add these values together.
- Divide by 100 to account for the percentage.
For instance, chlorine has two main isotopes: Cl-35 and Cl-37. About 75% of chlorine atoms are Cl-35 and about 25% are Cl-37.
To find the relative atomic mass (Ar) of chlorine, you would take 75% of the mass of Cl-35 and 25% of the mass of Cl-37 and add them together.
To find the weighted average for chlorine:
- Multiply the mass of each isotope by its abundance: (35 × 75) + (37 × 25)
- Add these values together: 2625 + 925 = 3550
- Divide by 100 to account for the percentage: 3550 ÷ 100 = 35.5
Relative atomic mass of chlorine = 35.5
Worked Example - RAM calculation using ratios
To find the weighted average using ratios:
- Multiply the mass of each isotope by its term (from the ratio).
- Add these values together.
- Divide by the total 'part's to account for the ratios.
Copper has two stable isotopes: Cu-63 and Cu-65. The ratio of Cu-63 to Cu-65 in nature is approximately 3:1. To find the relative atomic mass (Ar) of copper, you must use this ratio.
To find the weighted average for copper using ratios:
- Multiply the mass of each isotope by the number of parts: (63 × 3) + (65 × 1)
- Add these values together: 189 + 65 = 254
- Divide by the total number of parts to find the average: 254 ÷ 4 = 63.5
Relative atomic mass of copper = 63.5
Compounds
A compound is a substance made from two or more different elements' atoms that are chemically bonded together. There are three main types of chemical bonds:
- Ionic bonds: Formed between ions (which form when electrons are transferred from one atom to another).
- Covalent bonds: Formed when atoms share electrons.
- Metallic bonds: Formed between metal atoms, where electrons are free to move through the structure.
A chemical formula indicates the types and numbers of atoms in a molecule or compound. Examples include:
- water, H₂O - each molecule is made up of two hydrogen atoms and one oxygen atom
- sodium chloride, NaCl - each crystal contains one sodium ion for every one chlorine ion
- silicon dioxide, SiO₂ - for every silicon atom in this structure, there are two oxygen atoms
In chemical formulae, subscripts are used to show the number of each type of atom present. If no subscript is written, it is understood that there is only one atom of that element. For compounds containing polyatomic ions (brackets are often needed). Examples include:
- calcium hydroxide, Ca(OH)₂ - each calcium atom is bonded to two OH (hydroxide) groups (for every one calcium atom there are two oxygen atoms and two hydrogen atoms)
- magnesium nitrate, Mg(NO₃)₂ - each magnesium atom is bonded to two NO₃ (nitrate) groups (for every one magnesium atom there are two nitrogen atoms and six oxygen atoms)
- ammonium carbonate, (NH₄)₂CO₃ - there is one carbonate group (CO₃) for every two ammonium groups (NH₄) (for every one carbon atom there are three oxygen atoms, two nitrogen atoms, and eight hydrogen atoms)
When naming compounds, it’s essential to consider the elements involved and their positions in the Periodic Table. If the compound has more than one part to its name, such as sodium chloride, the element that is located furthest to the left in the Periodic Table is usually named first. This convention helps in systematically identifying and naming chemical compounds.
Compounds are often identified by their suffixes, which indicate the types of elements present and their bonding structures. Two common suffixes are:
- “-ide” is used for compounds consisting of only two elements: a metal and a non-metal. In these compounds, the metal’s name is mentioned first, followed by the non-metal’s name modified to end with “-ide.” For example, sodium fluoride (NaF) is composed of sodium (a metal) and fluorine (a non-metal).
- “-ate” is used for compounds that include three different elements: a metal, a non-metal, and oxygen. These compounds typically involve more complex bonding structures. An example is calcium carbonate (CaCO₃), which consists of calcium, carbon, and oxygen.
Worked Example - magnesium sulfide (MgS)
- Elements Involved: Magnesium (Mg) and Sulfur (S).
- Naming: The compound name ends with “-ide” because it consists of a metal (magnesium) and a non-metal (sulfur).
- Explanation: The name indicates a simple binary compound with no additional elements beyond the metal and non-metal.
Worked Example - magnesium sulfate (MgSO₄)
- Elements Involved: Magnesium (Mg), Sulfur (S), and Oxygen (O).
- Naming: The compound name ends with “-ate” because it includes a metal (magnesium), a non-metal (sulfur), and oxygen.
- Explanation: The “-ate” suffix denotes the presence of oxygen in the compound, in addition to the metal and non-metal, indicating a more complex molecular structure.
History of the Atom
Democritus (c. 460–370 BC), an ancient Greek philosopher, proposed that matter is made of tiny, indivisible particles called atoms. He believed that these atoms were eternal, indestructible, and varied in shape and size, which explained the different properties of materials. However, his ideas were purely philosophical and lacked experimental evidence.
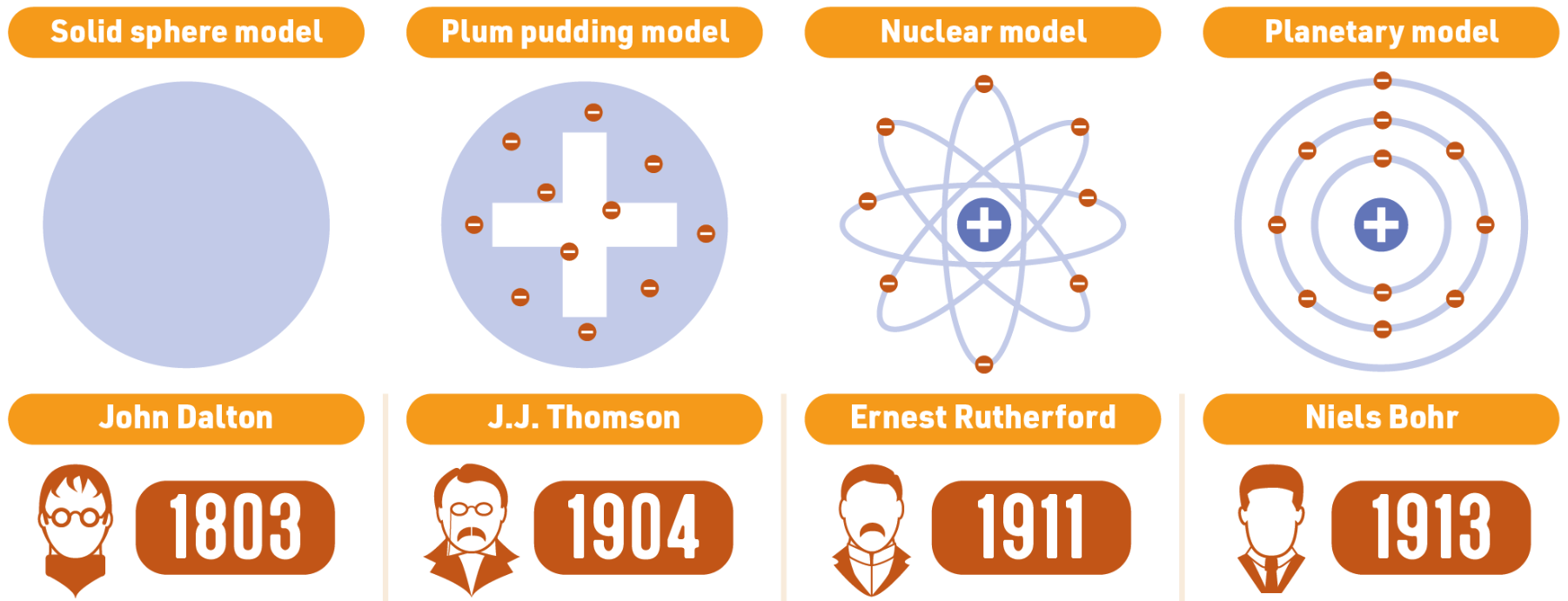
In 1803, John Dalton, an English chemist and physicist, revived the concept of atoms with his atomic theory. Dalton suggested that atoms are solid spheres and that different spheres make up different elements. He proposed that:
- All matter is made of atoms.
- Atoms of a given element are identical in mass and properties.
- Compounds are formed by a combination of two or more different kinds of atoms.
- A chemical reaction is a rearrangement of atoms.
J.J. Thomson (1897), a British physicist, discovered the electron through his experiments with cathode rays. He proposed the "plum pudding" model, where electrons were embedded in a positively charged "pudding." This model suggested that atoms were divisible and contained smaller, negatively charged particles (electrons) within a positively charged matrix.
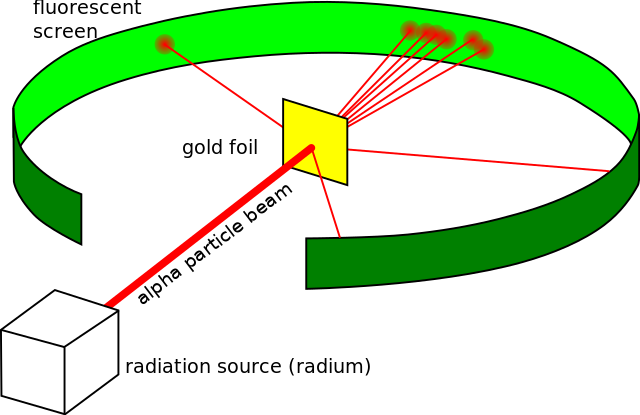
In 1911, Ernest Rutherford, a New Zealand-born physicist, discovered the nucleus through the famous gold foil experiment conducted by his lab assistants Hans Geiger and Ernest Marsden. In the gold foil experiment, a thin sheet of gold foil was bombarded with alpha particles. The key observations were:
- Most alpha particles passed straight through the foil.
- Some alpha particles were deflected at small angles.
- A few alpha particles were deflected back at large angles.
From these observations, Rutherford concluded that:
- Atoms are mostly empty space.
- The positive charge and most of the atom's mass are concentrated in a very small, dense region called the nucleus.
- The nucleus is positively charged and repels the positively charged alpha particles.
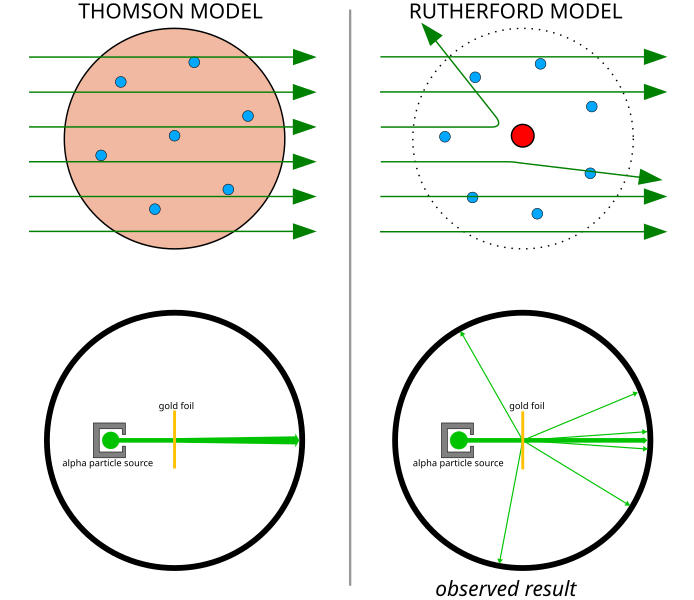
This led Rutherford to develop the nuclear model of the atom, where electrons orbit a central, positively charged nucleus.
In 1913, Niels Bohr, a Danish physicist, expanded on Rutherford's model by proposing that electrons orbit the nucleus at specific distances or energy levels. According to Bohr's model:
- Electrons travel in fixed orbits or shells around the nucleus.
- Each orbit has a specific energy level.
- Electrons can jump between energy levels by absorbing or emitting energy.
In 1932, James Chadwick, an English physicist and a student of Rutherford, discovered the neutron in 1932. Chadwick's discovery explained the existence of isotopes.
Listen to this page (feature coming soon)
Did you know?
- Hydrogen is the most abundant element in the universe, making up ~75% of the elemental mass.
- Hydrogen is the only element to have named isotopes (deuterium and tritium).
- The heaviest naturally occurring element is uranium, with an atomic number of 92.
Why do we care?
- Understanding atoms, elements, and compounds helps us grasp the basics of everything around us, from the air we breathe to the food we eat.
- This knowledge aids in making informed choices about nutrition, knowing the difference between elements like iron and compounds like iron supplements.
- It helps us understand the composition and properties of household products, leading to safer and more effective use.
- A strong foundation in these concepts is crucial for advanced studies and careers in science, engineering, and medicine.
Key information
- The modern atomic model consists of a nucleus (protons and neutrons) and electrons in electron shells.
- Isotopes are atoms of the same element with the same number of protons, but different numbers of neutrons.
- An element is a substance that consists of only one type of atom.
- Compounds are substances formed from two or more different elements chemically bonded together.
An isotope is an atom of the same element that has the same number of protons [1]
but a different number of neutrons. [1]
An atom consists of a central nucleus containing protons and neutrons. [1]
Electrons orbit the nucleus in electron shells. [1]
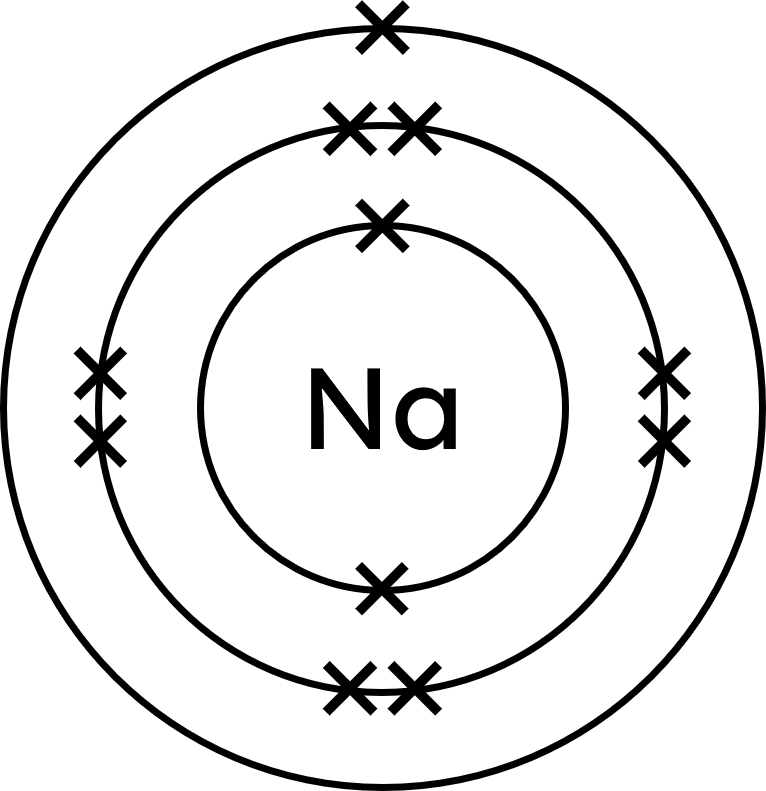
Sodium has an atomic number of 11, meaning it has 11 electrons. Therefore, the electron configuration of sodium is 2.8.1. [1]
Correct diagram as shown below [1]
The nuclear model:
- is mostly empty space [1]
- has all positive charge within the nucleus [1]
- has mass concentrated in the nucleus [1]
- has electrons orbiting the nucleus (they are separate) [1]
92.2% of silicon atoms have a mass of 28.
4.7% of silicon atoms have a mass of 29.
3.1% of silicon atoms have a mass of 30.
Ar = (28 × 0.922) + (29 × 0.047) + (30 × 0.031) [2]
= 25.816 + 1.363 + 0.93 = 28.109 [1]
rounded to suitable significant figures = 28.1 [1]