
Covalent bonding and structure (GCSE)
KS4 National Curriculum Statement(s) covered
- Types of chemical bonding: ionic, covalent, and metallic
- Bulk properties of materials related to bonding and intermolecular forces
- Changes of state of matter in terms of the relative strength of chemical bonds and intermolecular forces
- Structures, bonding, and properties of diamond, graphite, fullerenes, and graphene
Skip to:
Covalent bonding occurs when two non-metal atoms share pairs of electrons to achieve full outer shells. Each shared pair of electrons constitutes a covalent bond, resulting in the formation of molecules or giant covalent structures. Each atom must share 1 electron each to form one covalent bond - so each covalent bond is made of 2 electrons.
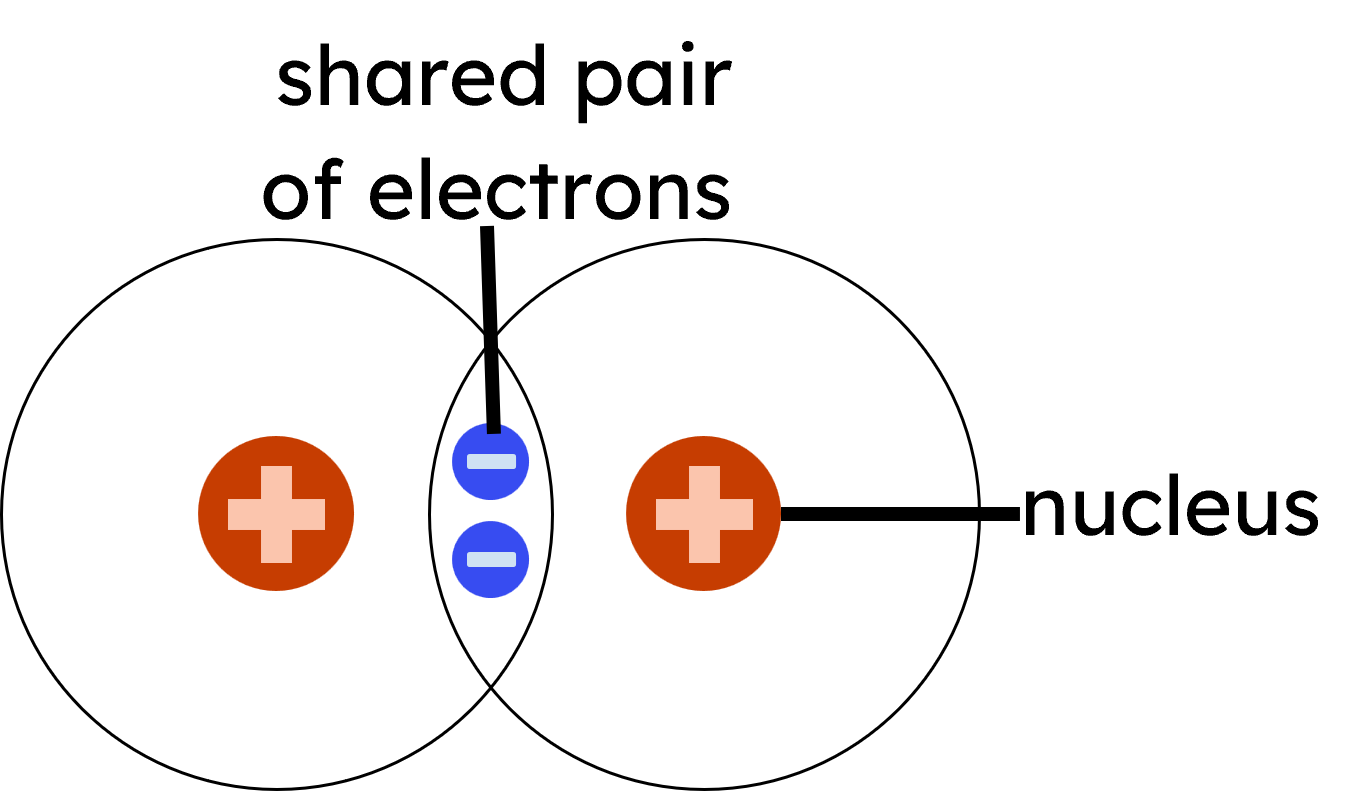
The term "covalent" comes from "co-" (together) and "valent" (related to valence electrons). The electrostatic attraction in covalent bonding is between the shared pair(s) of electrons and the nuclei of the bonded atoms.
Simple Molecular Structures

Simple molecular substances consist of small molecules held together by strong covalent bonds. They contain a discrete number of atoms within their structure, e.g. water (H₂O):
- Each hydrogen atom shares one electron with the oxygen atom.
- Oxygen shares one of its electrons with each hydrogen, forming two covalent bonds.

Simple molecules are typically around 0.1 nanometres in size.
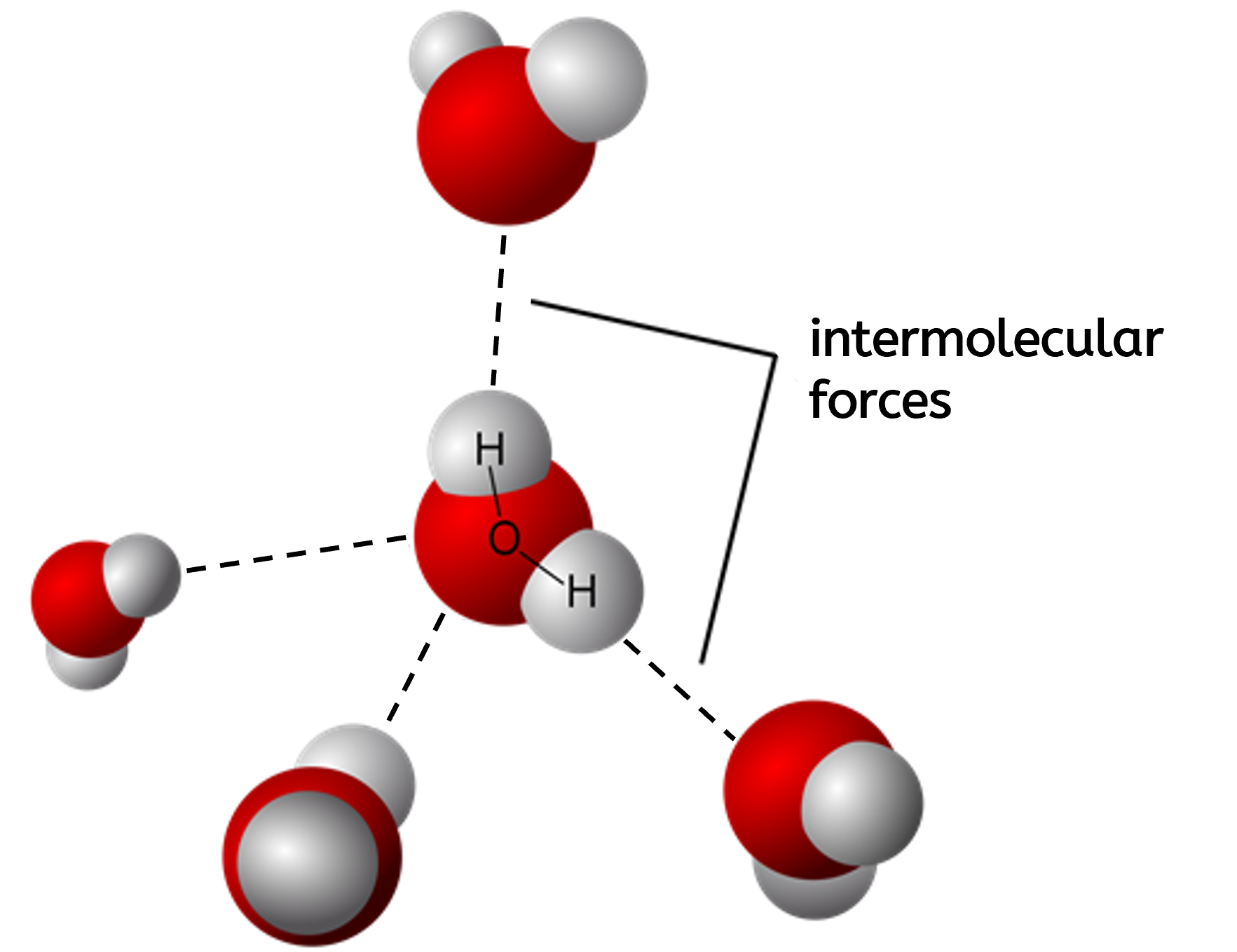
Properties of simple molecular substances:
- They have low melting and boiling points because little energy is required to overcome weak intermolecular forces of attraction between molecules
- They can be in the gas, liquid, or solid state depending on the strength of the intermolecular forces, but most are often in the liquid or gas state at room temperature and pressure
- They do not conduct electricity because there are no free-moving charge carriers (have neither delocalised electrons or free-moving ions)
- Most molecular substances are insoluble or only just soluble in water, but those that do dissolve often react with the water
Giant Covalent Structures
Giant covalent substances are made up of a vast number of atoms bonded together by covalent bonds, forming a giant lattice structure. Diamond, graphite and graphene are all giant structures that are allotropes of carbon.
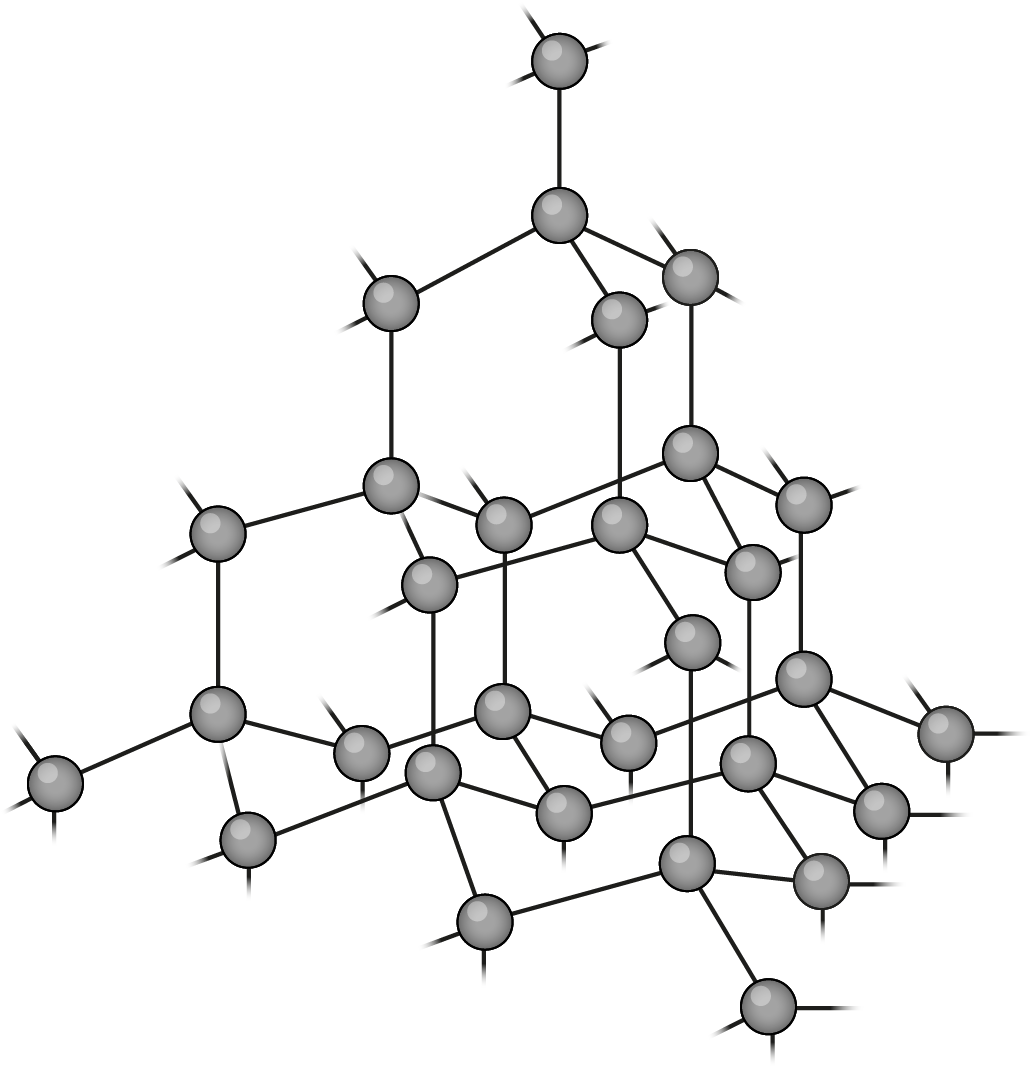
Diamond (C)
- An allotrope of carbon
- Each carbon atom forms four covalent bonds to other carbon atoms, creating a very hard, 3D tetrahedral lattice
- High melting point as lots of energy required to break strong covalent bonds between carbon atoms
- Does not conduct electricity (no free-moving electrons or ions)
- Diamond is the hardest natural material and is used in cutting tools

Graphite (C)
- An allotrope of carbon
- Each carbon atom forms three covalent bonds to other carbon atoms, creating layers of hexagonal rings
- High melting point as lots of energy required to break strong covalent bonds between carbon atoms
- Layers are held together by weak forces, allowing them to slide over each other
- Conducts electricity due to delocalised electrons
- Used in pencils and as a lubricant due to its layered structure

Graphene (C) is a single layer of graphite, where each carbon atom forms three covalent bonds to other carbon atoms, creating a single layer of hexagonal rings. It is:
- An allotrope of carbon
- Very strong
- Lightweight and flexible
- A good electrical conductor
- Used in electronics, sensors, and composite materials
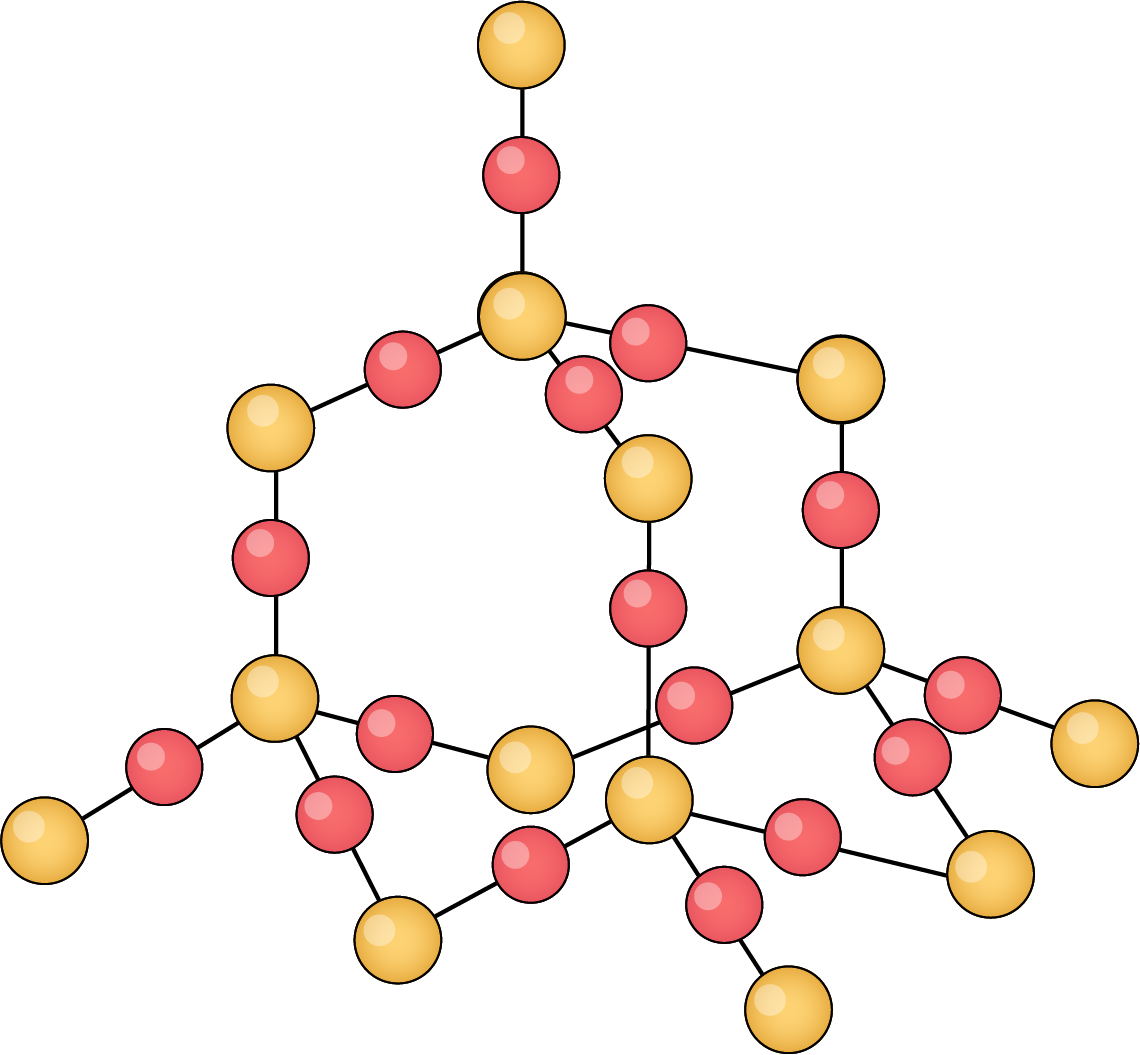
Silicon dioxide (SiO₂)
- Each silicon atom bonds with four oxygen atoms, creating a hard, 3D tetrahedral lattice
- High melting point as lots of energy required to break strong covalent bonds between silicon and oxygen atoms
- Found as quartz or in sand
Polymers

Polymers are large molecules made of repeating units called monomers, connected by covalent bonds. They are macromolecular structures, but not classed as giant covalent structures.
Polymers can be very long (thousands or millions of atoms). Their physical properties depend on the nature of the monomers and the conditions under which the polymer is made.
Poly(ethene) is made from repeating ethene (C₂H₄) units. It is a very widely used polymer, and is mostly used for plastic bags and bottles.
Fullerenes and Nanotubes
Fullerenes are molecules made entirely of carbon (another allotrope), taking the form of hollow spheres, ellipsoids, or tubes. A common fullerene is C₆₀ (buckminsterfullerene), which looks like a football and is sometimes called a buckyball.

Properties of spherical fullerenes:
- They have low melting points as little energy is needed to overcome the weak forces between molecules
- They have delocalised electrons within their structure, but these cannot pass from molecule to molecule (so cannot conduct electricity)
- They can act as lubricants as there are weak intermolecular forces between molecules, allowing them to slide around each other

Nanotubes, a type of cylindrical fullerene, have high length-to-diameter ratios and have a high tensile strength due to many strong covalent bonds. They can conduct electricity as delocalised electrons can move along the tube. Nanotubes are used in nanotechnology, electronics, and materials science.
Dot-and-Cross Diagrams
Dot-and-cross diagrams are a useful way to represent the transfer or sharing of electrons in chemical bonding. They show the outer shell electrons of atoms as crosses (×) or dots (•). This visual representation helps in understanding how atoms end up with full outer shells through bonding.
Only the outer shell electrons are involved in chemical bonding, so inner shells aren't often drawn for these diagrams.
Steps to draw dot-and-cross diagrams
- Determine the number of electrons in the outer shell of each atom.
- Decide whether the atoms will transfer or share electrons to achieve full outer shells.
- Decide which outer shell electrons of one atom will be represented with dots and which one with crosses.
- Draw the dot-and-cross diagram
- Double check each atom has access to 8 electrons (or 2 if it is hydrogen)
- For ionic compounds, include the charges on the resulting ions.
Worked Example - methane (CH₄)
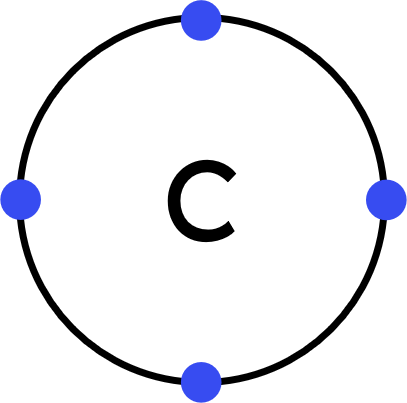
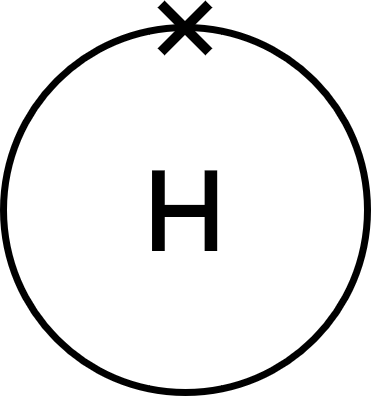
- Carbon (C) atoms have four electrons in their outer shell.
Each hydrogen (H) atom has one electron in its outer shell. - This is a covalent substance, so:
Carbon will share one electron with each of the four hydrogen atoms.
Each hydrogen will share one electron with carbon to achieve a full outer shell. - Represent the carbon atom's outer shell electrons with dots.
Represent each hydrogen atom's outer shell electrons with crosses. - Draw the covalent dot-and-cross:
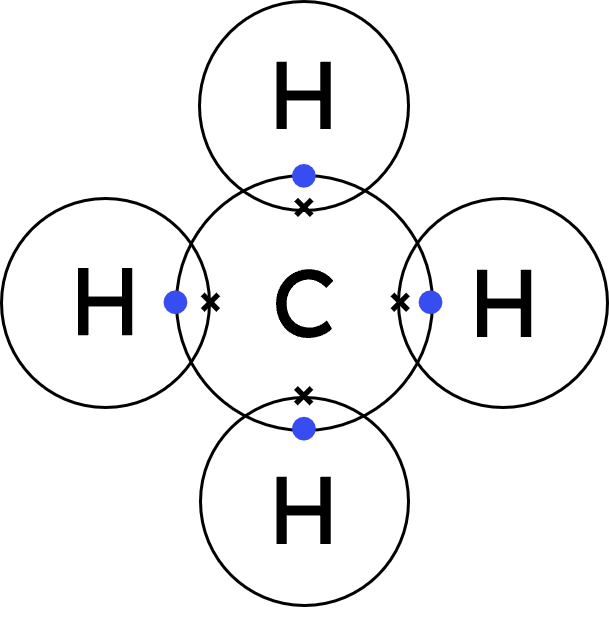
- The carbon atom has access to its original 4 outer shell electrons, and now one from each hydrogen atom (totalling 8).
Each hydrogen atom has access to its original outer shell electron, and one from the carbon atom (totalling 2).
Worked Example - oxygen (O₂)
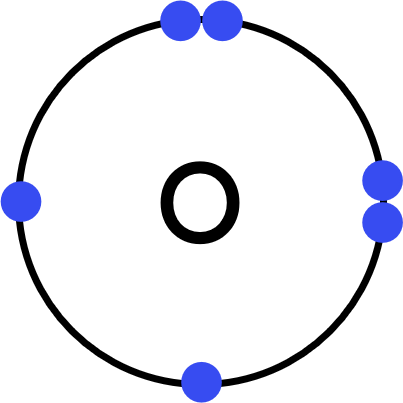

- Each oxygen (O) atom has six electrons in its outer shell.
- This is a covalent substance, so:
Each oxygen atom will share two electrons with the other oxygen to achieve a full outer shell. - Represent one oxygen's outer shell electrons with dots.
Represent the other oxygen's outer shell electrons with crosses. - Draw the covalent dot-and-cross:
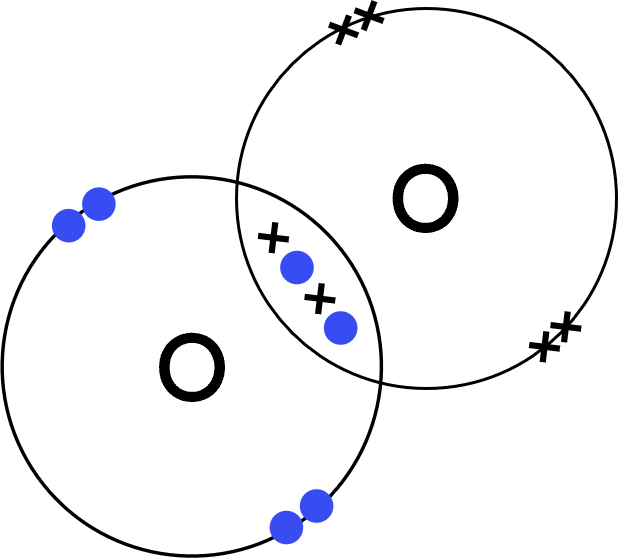
- Each oxygen atom has access to its original 6 electrons, and two from the other oxygen atom (totalling 8).
Bonding Models
| model | example | positives | limitations |
|---|---|---|---|
| chemical formula | NH₃ | shows how many atoms (either discrete - covalent; or ratio - ionic) | does not show which type of bonding, or the shape of molecule/lattice structure |
| displayed formula | %20(4).png?h=2964fbe41b18cae4c8358e66e19327a0) |
shows how atoms are connected in a molecule |
|
| dot-and-cross diagram | 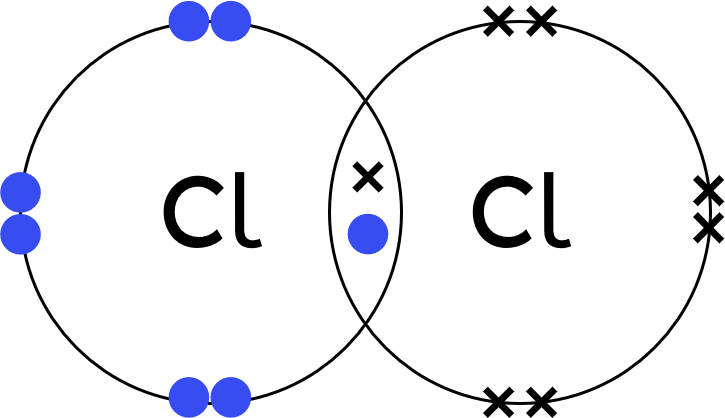 |
shows the sharing of electrons | does not show the 3D shape, relative sizes of atoms, or intermolecular forces |
| space-filling model | 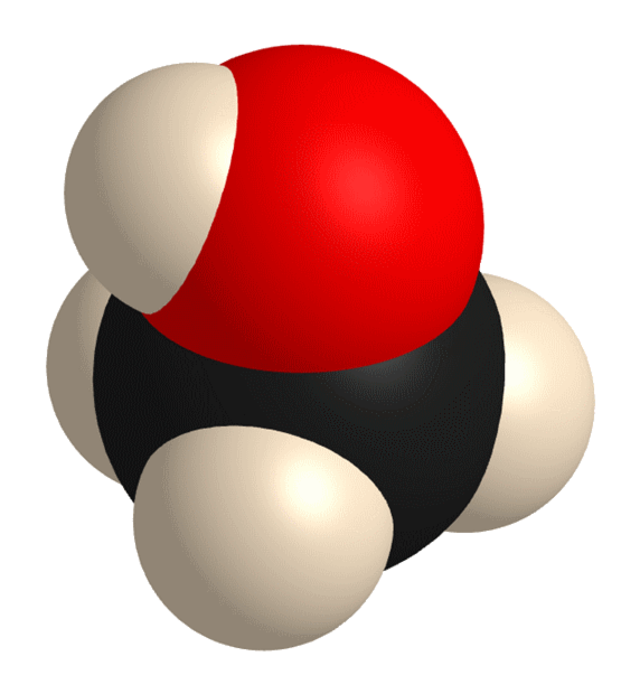 |
shows the 3D shape and relative sizes of atoms | does not show the sharing of electrons (how the bond forms), or which element is which (unless a key is given) |
| ball-and-stick model | 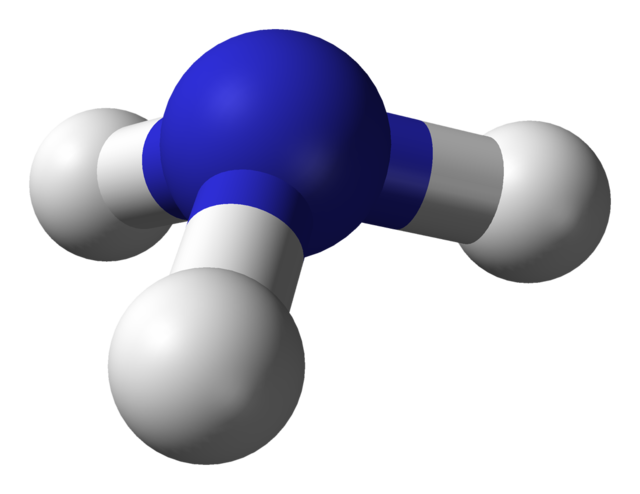 |
shows the 3D shape |
|
Listen to this page (feature coming soon)
Did you know?
- Silicones, used in waterproofing and lubricants, are polymers that consist of long chains of covalently bonded silicon and oxygen atoms.
- Graphene is not only incredibly strong but also flexible. Despite being just one atom thick, it can stretch up to 20% of its original length.
- Spider silk has a tensile strength comparable to steel and is composed of proteins with complex molecular structures. Its incredible strength and elasticity make it a subject of interest for developing new materials.
Why do we care?
- Understanding covalent bonding explains why water is liquid at room temperature and crucial for life processes, while carbon dioxide is a gas used in carbonated drinks.
- Learning about giant covalent structures like diamond and graphite helps us understand their use in cutting tools and as conductors in electronics.
- Knowing about polymers shows their role in everyday items like plastic bottles, clothing, and medical devices, highlighting the wide-ranging applications of covalent bonding.
- Knowledge of carbon nanotubes and fullerenes reveals their potential in future technologies, such as flexible electronics and high-strength materials, leading to advancements in various fields.
Key information
- Covalent bonding involves the sharing of electron pairs between atoms.
- Simple molecular substances, like water (H₂O) and carbon dioxide (CO₂), have low melting and boiling points due to weak intermolecular forces.
- Giant covalent structures, like diamond (C) and silicon dioxide (SiO₂), have high melting points because of strong covalent bonds throughout the structure.
- Graphite (C) conducts electricity due to the presence of free-moving electrons within its layers, unlike most covalent substances.