
Ionic bonding and structure (GCSE)
KS4 National Curriculum Statement(s) covered
- Types of chemical bonding: ionic, covalent, and metallic
- Bulk properties of materials related to bonding and intermolecular forces
- Changes of state of matter in terms of the relative strength of chemical bonds and intermolecular forces
Skip to:
Ionic bonding involves the electrostatic attraction between oppositely charged ions. This type of bonding typically occurs between metals and non-metals. Metal atoms lose electrons to form cations, while non-metal atoms gain electrons to form anions. The electrostatic attraction between these ions forms a strong bond and creates a regular, repeating lattice structure.
Forming Ions
Ions are atoms (or groups of atoms) that have lost or gained electrons and thus carry a charge. Metal atoms lose electrons to form positively charged ions (cations), while non-metal atoms gain electrons to form negatively charged ions (anions).
The Periodic Table is a handy helping guide as to which elements' atoms will gain or lose electrons, when forming ions:
| group number | example element | outershell electrons | electrons lost or gained | ion formed |
|---|---|---|---|---|
| 1 | sodium (Na) | 1 | lose 1 | 1+ |
| 2 | magnesium (Mg) | 2 | lose 2 | 2+ |
| 3 | aluminium (Al) | 3 | lose 3 | 3+ |
| 4 | silicon (Si) | 4 | N/A | N/A |
| 5 | phosphorus (P) | 5 | gain 3 | 3- |
| 6 | sulfur (S) | 6 | gain 2 | 2- |
| 7 | chlorine (Cl) | 7 | gain 1 | 1- |
| 0 | argon (Ar) | 8 | already full/stable | N/A |
Examples:
- Sodium (Na, Group 1) loses one electron to form an Na⁺ ion
- Sulfur (S, Group 6) gains two electrons to form a S²⁻ ion
| ion name | ion symbol/formula | example compound |
|---|---|---|
| oxide | O²⁻ | magnesium oxide (MgO) |
| hydroxide | OH⁻ | lithium hydroxide (LiOH) |
| halide | F⁻, Cl⁻, Br⁻, I⁻ | sodium chloride (NaCl) |
| nitrate | NO₃⁻ | potassium nitrate (KNO₃) |
| carbonate | CO₃⁻ | calcium carbonate (CaCO₃) |
| sulfate | SO₄²⁻ | barium sulfate (BaSO₄) |
Ionic Structure
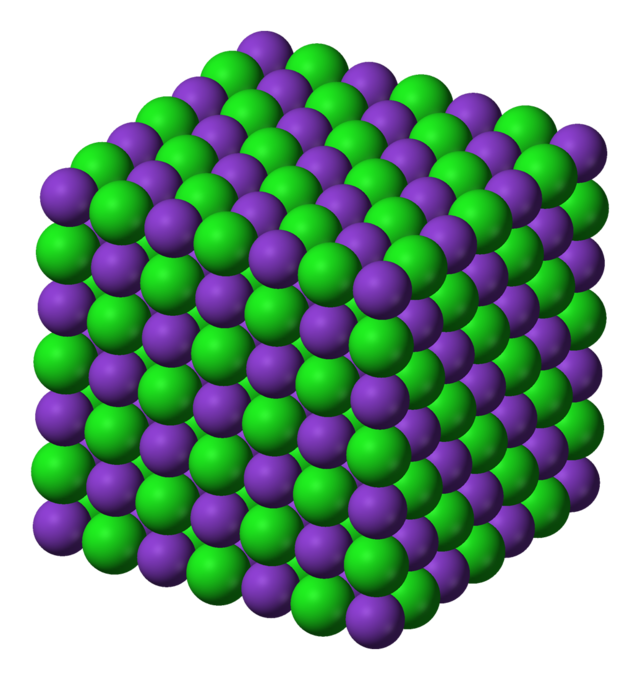
Ionic substances are considered "giant" substances because they form extensive 3D lattice structures composed of repeating units of positive and negative ions. The strong electrostatic forces of attraction between these oppositely charged ions extend throughout the entire substance.
In a giant ionic lattice, each ion is surrounded by ions of the opposite charge, and the electrostatic forces of attraction act in all directions. This uniform attraction results in strong, stable structures.
Properties of ionic compounds:
- They have high melting and boiling points because the strong electrostatic forces require a lot of energy to break
- They conduct electricity when molten or dissolved in water because the ions are free to move and carry charge
- They do not conduct electricity when solid because the ions are fixed in place and cannot move
- Some ionic substances are water soluble, depending on the attraction between the ions and water molecules
In this animated gif, a water-soluble salt is added to water. As the salt dissolves, its ions separate and disperse throughout the water. This happens because the water molecules surround and interact with the ions, pulling them apart and overcoming the forces of attraction that hold the ions together in the solid state. This process, called dissociation, allows the salt to dissolve completely in the water.

However, not all ionic substances are soluble in water. If the forces of attraction between the ions in the solid are too strong, water molecules cannot pull them apart, and the substance will not dissolve.
Dot-and-Cross Diagrams
Dot-and-cross diagrams are a useful way to represent the transfer or sharing of electrons in chemical bonding. They show the outer shell electrons of atoms as crosses (×) or dots (•). This visual representation helps in understanding how atoms end up with full outer shells through bonding.
Only the outer shell electrons are involved in chemical bonding, so inner shells aren't often drawn for these diagrams. Ionic dot-and-cross diagrams, however, do require you to draw the new, 'revealed' outer shell for metal ions (after they have given their outer shell electrons to the non-metal atom).

Steps to draw dot-and-cross diagrams
- Determine the number of electrons in the outer shell of each atom.
- Decide whether the atoms will transfer or share electrons to achieve full outer shells.
- Decide which outer shell electrons of one atom will be represented with dots and which one with crosses.
- Draw the dot-and-cross diagram
- Double check each atom has access to 8 electrons (or 2 if it is hydrogen)
- For ionic compounds, include the charges on the resulting ions.
Worked Example - sodium chloride (NaCl)
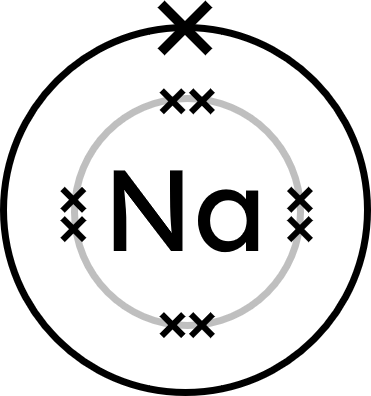
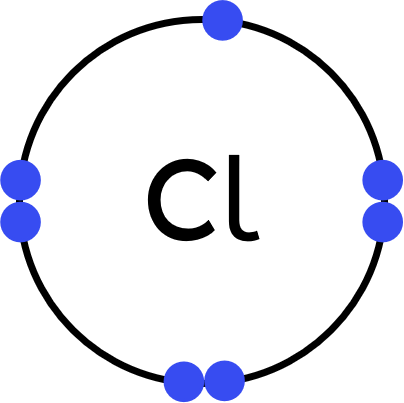
- Sodium (Na) atoms have one electron in their outer shell.
Chlorine (Cl) atoms have seven electrons in their outer shell. - This is an ionic substance, so:
Sodium will lose one electron to achieve a full outer shell, becoming Na⁺.
Chlorine will gain one electron to achieve a full outer shell, becoming Cl⁻. - Represent sodium's new, relealed outer shell electrons with dots.
Represent chlorine's outer shell electrons with crosses, plus one dot for the gained electron. - Draw the ionic dot-and-cross:
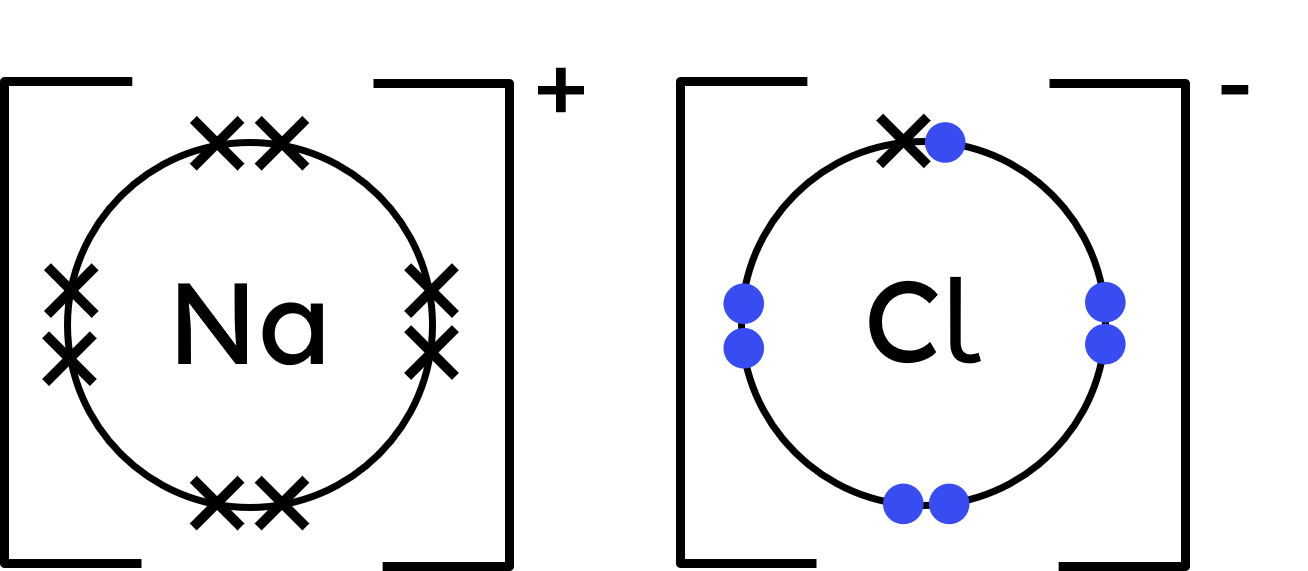
- The sodium atom 'revealed' a full shell of electrons (8) when it became a sodium ion.
The chloride ion has access to its original 7 electrons, and now one more from sodium (totalling 8).
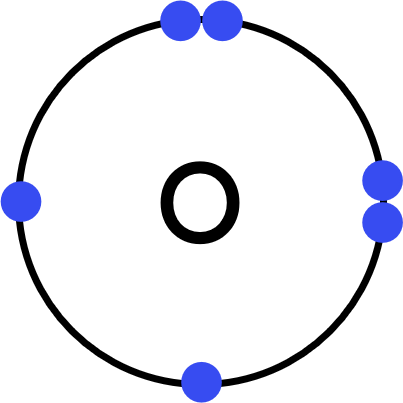
Worked Example - aluminium oxide (Al₂O₃)
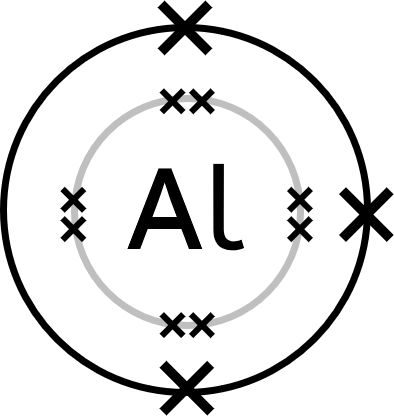
- Aluminium (Al) atoms have three electrons in their outer shell.
Oxygen (O) atoms have six electrons in their outer shell. - This is an ionic substance, so:
Two aluminium atoms will each lose three electrons to form two Al³⁺ ions.
Three oxygen atoms will each gain two electrons to form three O²⁻ ions. - Represent aluminium's new, revealed outer shell electrons with dots.
Represent oxygen's outer shell electrons with crosses, plus two dots for the gained electrons. - Draw the ionic dot-and-cross:
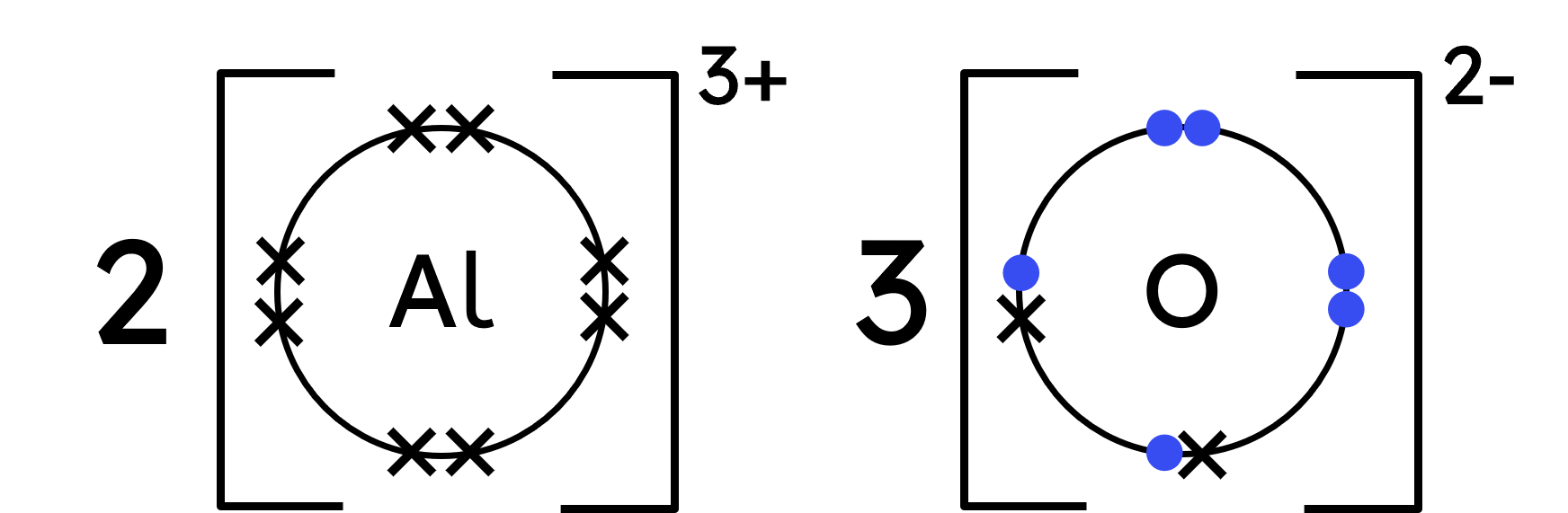
- Each aluminium atom 'revealed' a full shell of electrons (8) when they became aluminium ions.
The oxide ion has access to its original 6 electrons, and now two more from aluminium (totalling 8).
Bonding Models
| model | example | positives | limitations |
|---|---|---|---|
| chemical formula | NaCl | shows how many atoms (either discrete - covalent; or ratio - ionic) | does not show which type of bonding, or the shape of molecule/lattice structure |
| dot-and-cross diagram |  |
shows the transfer of electrons and the charges on ions | does not show the 3D shape or relative sizes of ions, or the uniform nature of ionic bonding |
| space-filling model |  |
shows the 3D shape and relative sizes of atoms | does not show how ions were formed or the charges on ions, or which element is which (unless a key is given) |
| ball-and-stick model |  |
shows the 3D shape |
|
Listen to this page (feature coming soon)
Did you know?
- Sea shells are made of calcium carbonate, which is an example of a compound formed through ionic bonding.
- Table salt (sodium chloride) is not just used for seasoning food; it’s also used to de-ice roads in winter.
- Ionic compounds are typically brittle; they shatter when a force is applied due to the repulsion between like-charged ions.
Why do we care?
- Understanding how ions form explains why substances like table salt dissolve in water, which is crucial for processes like cooking and maintaining body fluids.
- Knowing the structure of ionic compounds, such as the strong lattice structure in sodium chloride, helps us understand why these materials are used in road gritting and food preservation.
- Dot-and-cross diagrams make it easier to visualise ionic bonding, helping students grasp concepts needed for further studies in chemistry and practical applications like creating new materials.
- Bonding models of ionic substances are essential for predicting their properties, aiding in the development of products like durable ceramics, effective medications, and efficient batteries.
Key information
- Ionic substances form through the transfer of electrons from a metal to a non-metal, creating positive and negative ions.
- Ionic compounds form giant lattice structures, resulting in high melting and boiling points.
- Ionic substances conduct electricity when molten or dissolved in water because ions are free to move.