
Metallic bonding and structure (GCSE)
KS4 National Curriculum Statement(s) covered
- Types of chemical bonding: ionic, covalent, and metallic
- Bulk properties of materials related to bonding and intermolecular forces
- Changes of state of matter in terms of the relative strength of chemical bonds and intermolecular forces
Skip to:
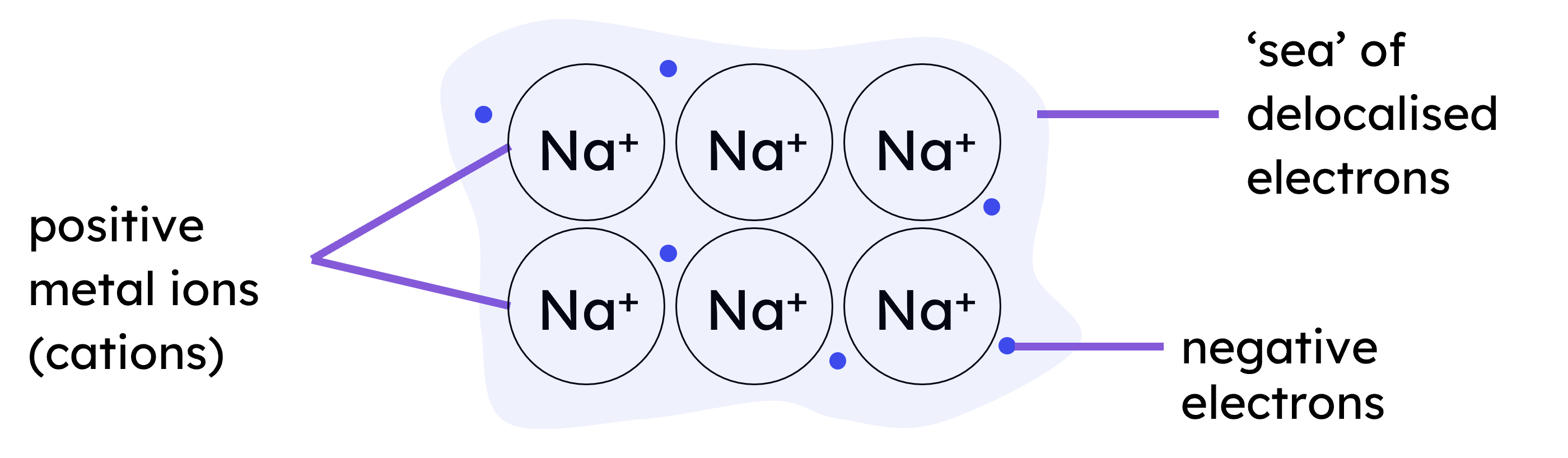
Metallic bonding occurs in metals, where their atoms lose the electrons from their outer shells. These electrons become delocalised, forming a "sea of electrons" that move freely around the positive metal ions. This creates a strong electrostatic attraction between the positive ions and the delocalised electrons, holding the metal together in a giant metallic structure.
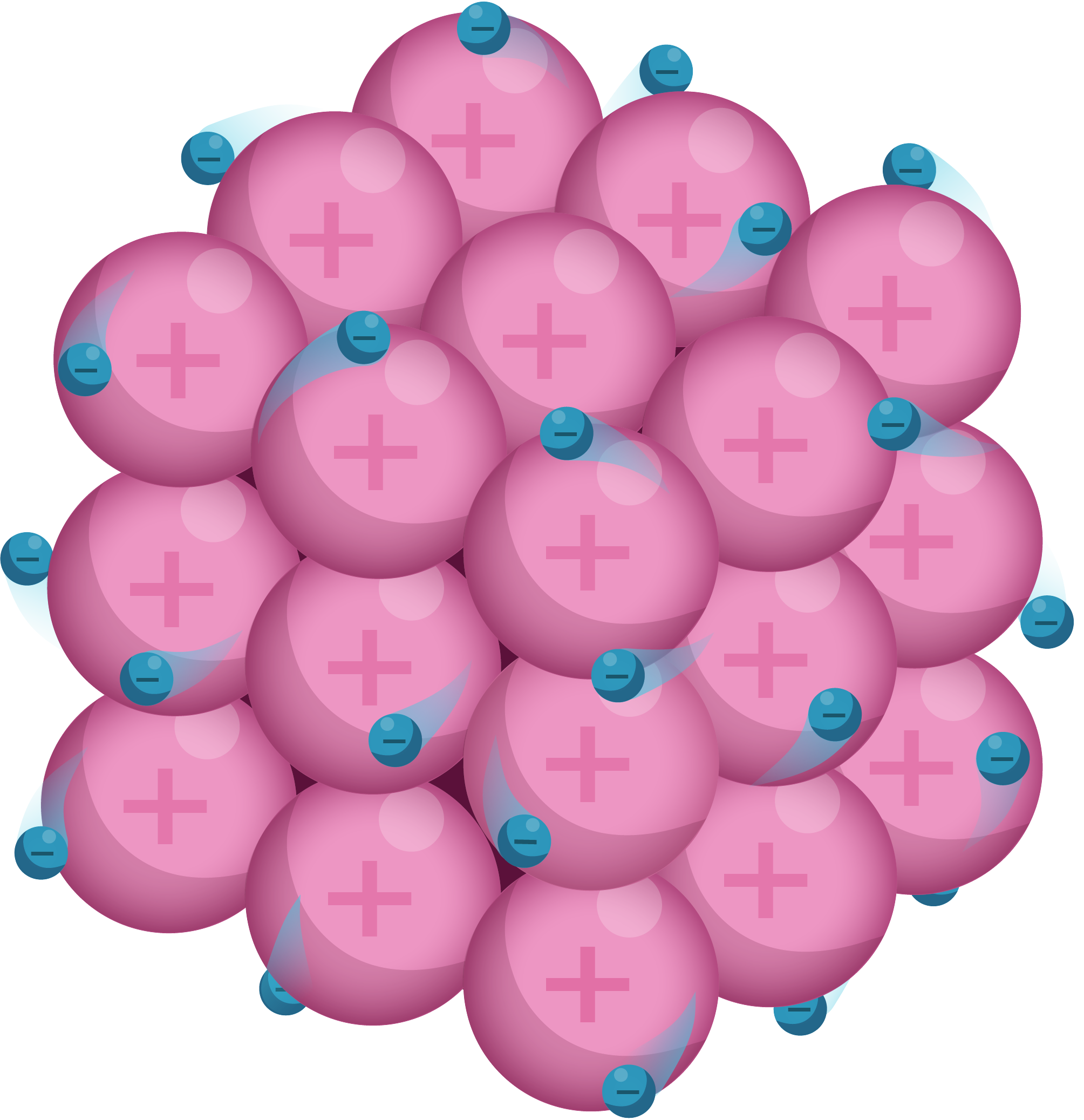
Forming Ions
Ions are atoms (or groups of atoms) that have lost or gained electrons and thus carry a charge. Metal atoms lose electrons to form positively charged ions (cations). The Periodic Table is a handy guide to understanding which metal atoms will lose electrons to form cations, when forming ions:
| group number | example element | outershell electrons | electrons lost or gained | ion formed |
|---|---|---|---|---|
| 1 | sodium (Na) | 1 | lose 1 | 1+ |
| 2 | magnesium (Mg) | 2 | lose 2 | 2+ |
| 3 | aluminium (Al) | 3 | lose 3 | 3+ |
Examples:
- Sodium (Na, Group 1) loses one electron to form an Na⁺ ion
- Calcium (Ca, Group 2) loses two electrons to form a Ca²⁺ ion.
Properties and uses
Common properties of metals:
- High melting and boiling points: Strong electrostatic forces require a lot of energy to break.
- Good conductors of electricity and heat: Delocalised electrons can move freely and carry charge.
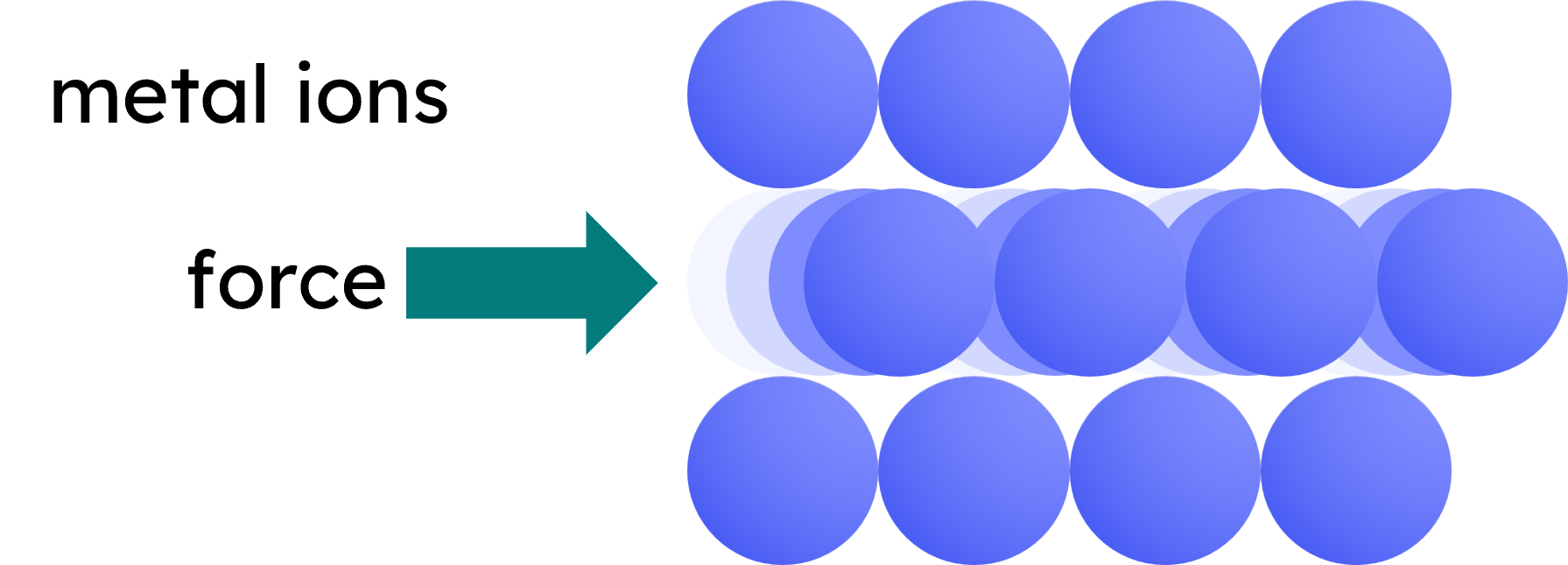
- Malleable and ductile: Layers of atoms can slide over each other without breaking the metallic bonds.
In pure metals, the atoms are arranged in a regular lattice structure. This regular arrangement allows the layers of atoms to slide over each other easily when a force is applied, making the metals malleable.
Aluminium, gold and copper are useful metals, and the uses of these metals is related to their properties. Aluminium does not react with water, as its surface is protected by a naturally formed layer of aluminium oxide (allows the metal to resist corrosion). Aluminium foil is used domestically to wrap/store food, because:
- it does not react with the substances in food
- it is malleable and so can easily be folded around the food
- it has a low density so it is very lightweight
Alloys
In pure metals, atoms are arranged in layers, which allows metals to be bent and shaped. This structure gives pure metals their malleability, meaning they can be easily deformed without breaking. However, this property also makes them too soft for many practical uses. To increase their hardness and utility, pure metals are often mixed with other metals or elements to form alloys.
Alloys are mixtures of metals with other metals or non-metal elements, which disrupt the regular arrangement of atoms (as the atoms are different sizes) meaning the layers can no longer slide over each other as easily. This is often done to enhance the properties of the base metal, such as increase strength and resistance to corrosion (and reduce malleability).
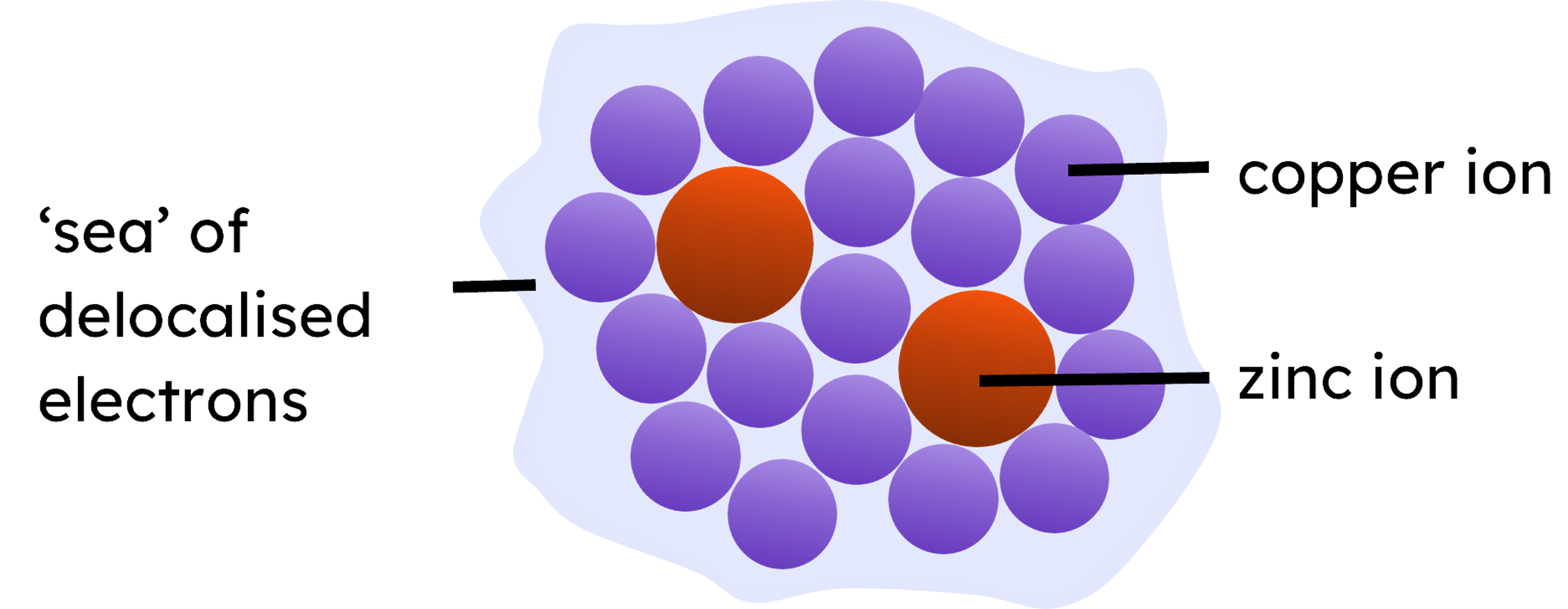
Alloys are mixtures of metals with other metals or non-metal elements, which disrupt the regular arrangement of atoms (as the atoms are different sizes) meaning the layers can no longer slide over each other as easily. This is often done to enhance the properties of the base metal, such as increase strength and resistance to corrosion (and reduce malleability).
Most metals in everyday use are alloys. Some examples include:
| alloy | composition | properties | use |
|---|---|---|---|
| bronze | copper and tin | hard, wear-resistant | statues, bearings |
| brass | copper and zinc | corrosion-resistant, easy to cast | musical instruments, fittings |
| jewellery gold | gold, silver, copper, zinc | harder than pure gold, but still malleable | jewellery |
| high carbon steel | iron and high carbon | strong, but brittle | cutting tools, springs |
| low carbon steel | iron and low carbon | soft, easily shaped | car body parts |
| stainless steel | iron, chromium, nickel | hard, resistant to rusting | cutlery, surgical instruments |
| magnalium | aluminium and magnesium | lightweight, strong | aircraft parts |
Gold used in jewellery is usually alloyed with silver, copper, and zinc to increase its hardness. The proportion of gold in these alloys is measured in carats, with 24 carat being pure gold and 18 carat being 75% gold.
Aluminium alloys are known for their low density, making them suitable for applications where weight reduction is important.
Each alloy has specific properties tailored to its intended use. For instance, stainless steel's resistance to rusting makes it ideal for cutlery and surgical instruments, while the low density of magnalium makes it suitable for aircraft parts. Understanding the composition and properties of these alloys helps in selecting the right material for various applications.
Listen to this page (feature coming soon)
Did you know?
- The Eiffel Tower can be 15 cm taller during the summer because metal expands when it gets hot.
- Gold is so ductile that one gram can be drawn into a thread over two kilometres long.
- Gallium is a metal that can melt in your hand since it has a melting point of about 30°C.
Why do we care?
- Understanding how cations form in metals helps explain why metals can conduct electricity and heat, which is crucial for electrical wiring and cookware.
- Knowing the properties of metals, such as malleability and ductility, explains their use in construction, manufacturing, and making items like aluminium foil and copper pipes.
- Learning about alloys, such as stainless steel and bronze, shows their importance in creating materials with enhanced properties for specific uses, like cutlery and musical instruments.
- Learning about alloys, which are mixtures of metals, shows their importance in creating materials with specific properties, like stainless steel for cutlery and aluminium alloys for aircraft.
Key information
- Metallic bonding involves a lattice of positive metal ions surrounded by a sea of delocalised electrons.
- Metals are good conductors of electricity and heat due to the mobility of delocalised electrons.
- Metals are malleable and ductile because the layers of atoms can slide over each other while maintaining the metallic bond.
- Alloys, which are mixtures of metals, can have enhanced properties such as increased strength and resistance to corrosion.