Chemical reactions (GCSE)
KS4 National Curriculum Statement(s) covered
- Balanced chemical equations, ionic equations, and state symbols
- Measurement of energy changes in chemical reactions (qualitative)
- Bond breaking, bond making, activation energy, and reaction profiles (qualitative)
Skip to:
Chemical reactions are processes where substances, known as reactants, are transformed into different substances, called products. This transformation is achieved through the breaking and forming of chemical bonds. Chemical reactions are foundational to the study of chemistry and play a vital role in both natural processes and industrial applications.
The history of chemical reactions dates back to ancient times when early chemists, known as alchemists, sought to transform base metals into gold and discover the elixir of life. These early efforts, while often mystical, laid the groundwork for modern chemistry.
In the 17th century, Robert Boyle showed that experiments and the scientific method were very important. His work helped change chemistry from the mystical ideas of alchemy to a more evidence-based science. The 18th century saw significant advancements with Antoine Lavoisier, often referred to as the father of modern chemistry. Lavoisier discovered the role of oxygen in combustion and established the law of conservation of mass, which states that mass is neither created nor destroyed in a chemical reaction.
Chemical reactions can often be classified in more than one way, depending on the criteria used for classification. A single reaction might fall into multiple categories, highlighting different aspects of the process involved.
The combustion of methane is a good example of a reaction that can be classified in several ways. When methane (CH₄) reacts with oxygen (O₂), it produces carbon dioxide (CO₂) and water (H₂O), releasing energy in the form of heat and light.
methane + oxygen → carbon dioxide + water
CH₄ + O₂ → CO₂ + H₂O
- This reaction is classified as a combustion reaction because it involves the burning of methane in the presence of oxygen.
- The reaction is also an exothermic reaction because it releases energy to the surroundings.
- This is also a redox reaction, because in this process the carbon in methane is oxidised, and oxygen is reduced.
By understanding these different classifications, chemists can gain a more comprehensive understanding of the chemical processes involved. Each classification provides unique insights into the reaction mechanisms, energy changes, and electron transfers that occur during the reaction.
Learn more about chemical reactions by clicking on the links below:
Observations
Observing chemical reactions and distinguishing them from physical changes is a key skill in chemistry. Chemical reactions involve the transformation of substances into new products, often accompanied by observable changes such as:
- energy change
- colour change
- gas production
- solid substance forms
- change in smell
In contrast, physical changes do not alter the chemical identity of a substance. These changes involve alterations in the state or appearance of a substance but do not result in new substances. Examples include melting, freezing, and dissolving. For instance, when ice melts to form water, it undergoes a physical change. The molecular structure of water remains the same, with H₂O molecules simply moving from a solid state to a liquid state.
| observation | description | chemical reaction examples | physical change examples |
|---|---|---|---|
| energy change | light or sound produced during a reaction (or temperature increase/ decrease) | combustion of fuel (releases heat and light, forms carbon dioxide and water); calcium oxide with water (releases heat, forms calcium hydroxide) | melting ice (temperature increase) |
| colour change | a change in visual appearance (colour) of a substance | rusting of iron (grey to reddish-brown, forms iron oxide); reaction between phenolphthalein and a base (colourless to pink) | heating metal until it glows red |
| gas production | formation of gas bubbles | effervescence from vinegar and baking soda (produces carbon dioxide); bubbles of oxygen gas from decomposition of hydrogen peroxide | boiling water (produces water vapour bubbles) |
| solid substance forms | a solid substance appears (when there wasn't one before) | lead iodide is produced from adding lead nitrate solution to potassium iodide solution (forms yellow lead iodide precipitate) | freezing water (solid ice forms); crystallisation of salt from a solution (salt crystals form) |
| change in smell | a new or different smell | smell of rotten eggs from the reaction of iron sulfide with hydrochloric acid (FeS + 2HCl → FeCl₂ + H₂S) | smell of perfume from evaporation (perfume smell spreads) |

When a piece of magnesium ribbon burns in air, it reacts with oxygen to form magnesium oxide. This reaction is evident through the bright white light produced and the formation of a new white powdery substance, indicating a chemical change.

Conversely, dissolving sugar in water is a physical change. The sugar molecules disperse within the water, but no new substances are formed, and the process can be reversed by evaporating the water to retrieve the sugar.
Word Equations
Word equations provide a straightforward way to describe chemical reactions using words instead of symbols. They are particularly useful for conveying the reactants and products involved in a reaction without delving into the specifics of chemical formulae and balancing.
In any chemical reaction, the starting substances are known as reactants, and the substances formed as a result of the reaction are called products.
Reactants:
- These are the substances you start with in a chemical reaction.
- They undergo changes during the reaction.
Products:
- These are the new substances formed as a result of the chemical reaction.
- They have different properties and compositions compared to the reactants.
In chemical equations, we use the arrow symbol (→) instead of the equals sign (=) to represent the direction of the chemical reaction. This distinction is important as:
- The arrow (→) indicates the direction in which the reaction proceeds. It shows that the reactants on the left side are transformed into products on the right side.
- This directional arrow highlights the process of change, unlike the equals sign, which implies a static, unchanging relationship between the two sides.
- Many chemical reactions are not easily reversible under standard conditions. The arrow symbol effectively communicates that the reaction predominantly goes in one direction.
Consider the reaction between hydrogen and oxygen to form water. The word equation for this reaction is:
hydrogen + oxygen → water
This equation tells us that hydrogen and oxygen are the reactants, and water is the product. It does not, however, provide information on the quantities or states of these substances.
The reaction between magnesium and hydrochloric acid can be represented as:
magnesium + hydrochloric acid → magnesium chloride + hydrogen
In this reaction, magnesium reacts with hydrochloric acid to produce magnesium chloride and hydrogen gas. Here, magnesium and hydrochloric acid are the reactants, while magnesium chloride and hydrogen are the products.
These word equations describe the reactants and products but do not provide information about the quantities or the specific chemical forms of the substances. They are useful for getting a general understanding of the reaction process.
Balanced Symbol Equations
Balanced symbol equations provide a detailed representation of chemical reactions, showing the exact quantities of reactants and products involved. These equations adhere to the law of conservation of mass, ensuring that the number of atoms of each element is the same on both sides of the equation.
State symbols provide additional information about the physical state of the reactants and products. They are placed in brackets after the chemical formulae:
- solid (s)
- liquid (l)
- gas (g)
- aqueous (aq)
| equation | reaction 1 | reaction 2 |
|---|---|---|
| word | hydrogen + oxygen → water | iron + copper sulfate → iron sulfate + copper |
| unbalanced symbol | H₂ + O₂ → H₂O | Fe + CuSO₄ → FeSO₄ + Cu |
| balanced symbol | 2H₂ + O₂ → 2H₂O | already balanced |
| balanced with state symbols | 2H₂ (g) + O₂ (g) → 2H₂O (l) | Fe (s) + CuSO₄ (aq) → FeSO₄ (aq) + Cu (s) |
A common misconception is that balancing an equation involves manipulating the chemical reaction itself. However, this is not the case. The purpose of balancing a chemical equation is to ensure that the same number of each type of atom appears on both sides of the equation. This balance reflects the reality that atoms are neither created nor destroyed in a chemical reaction; they are simply rearranged. This principle is known as the law of conservation of mass.
When chemists write a chemical equation, they are representing the actual chemical formulae of the reactants and products. These formulae are fixed and cannot be changed. For instance, water (H₂O) always consists of two hydrogen atoms and one oxygen atom. This ratio is inherent to the substance and is represented by the subscript numbers in the formula. Changing these subscript numbers would mean describing a different chemical substance altogether, which is incorrect as it would not be balancing the equation (you'd be writing a different equation altogether).
Balancing equations is essential because an unbalanced equation cannot accurately describe the reality of the reaction. It fails to account for the correct quantities of substances involved, making it impossible to use for predicting the outcomes of reactions or for practical applications like laboratory experiments or industrial processes.
To balance a chemical equation, follow these steps:
- First, write the unbalanced equation using chemical symbols and formulae.
- Next, count the number of atoms of each element on both sides of the equation.
- Adjust the coefficients (the numbers in front of the chemical formulae) to balance the atoms, ensuring that the same number of each type of atom appears on both sides.
- Add state symbols to show which physical state each substance is in during the reaction.
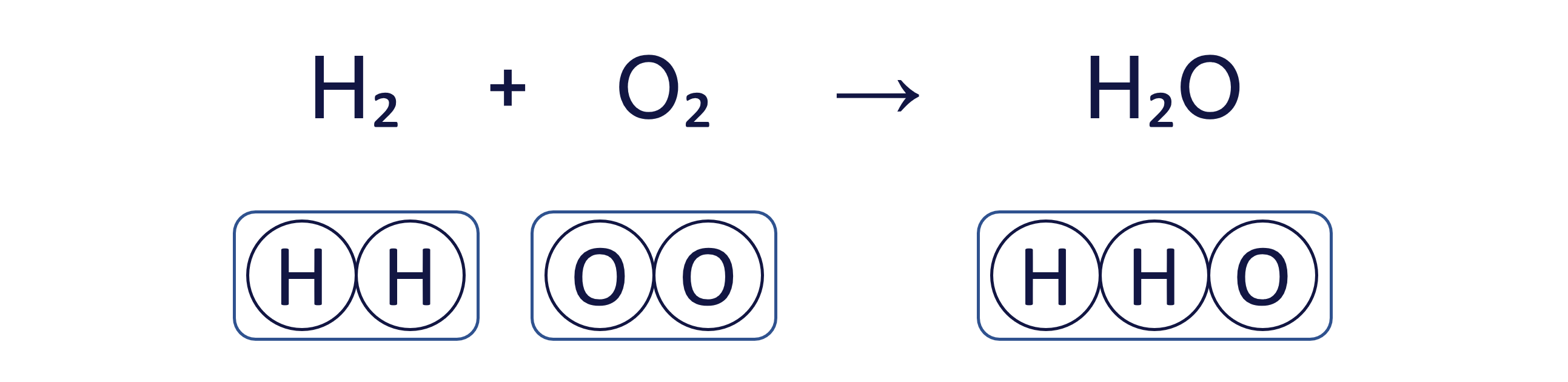
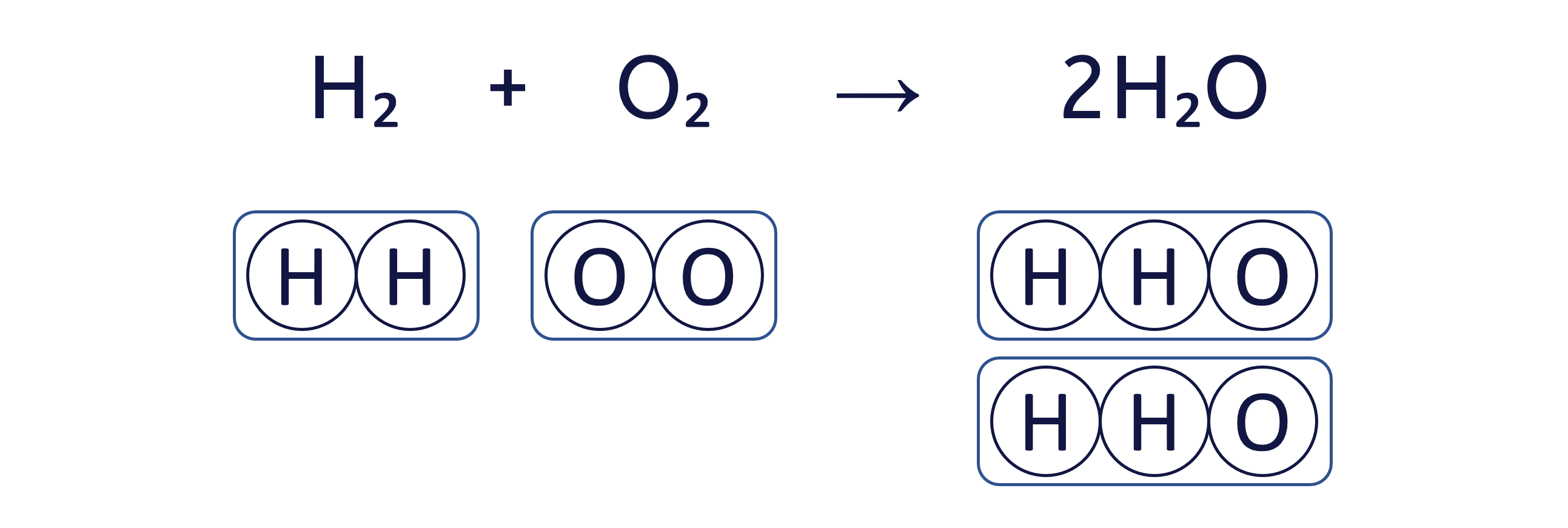
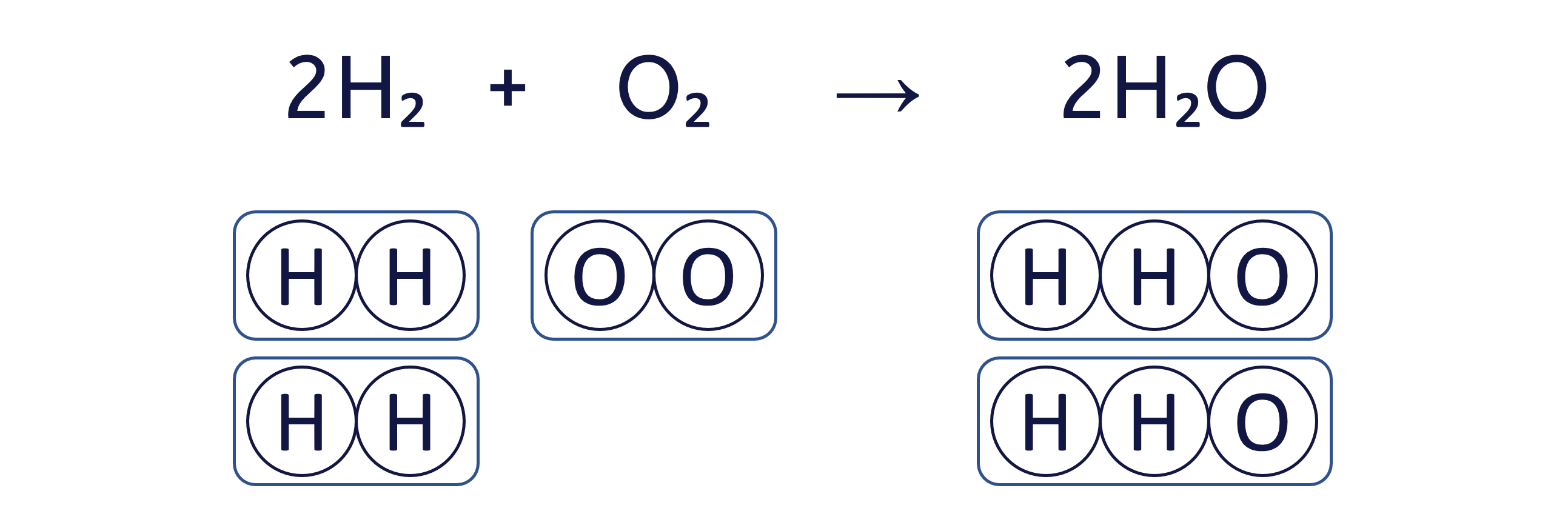
Worked Example - Balancing equations (beginner)
To balance an equation, you must adjust the coefficients (the numbers placed before the chemical formulae) to ensure that the quantity of each element is the same on both sides.
The box method visually ensures that we do not change the formula of any substance, only the quantity of each 'box' (substance) to balance the equation.
To balance the chemical equation H₂ + O₂ → H₂O using the box method, follow these three steps:
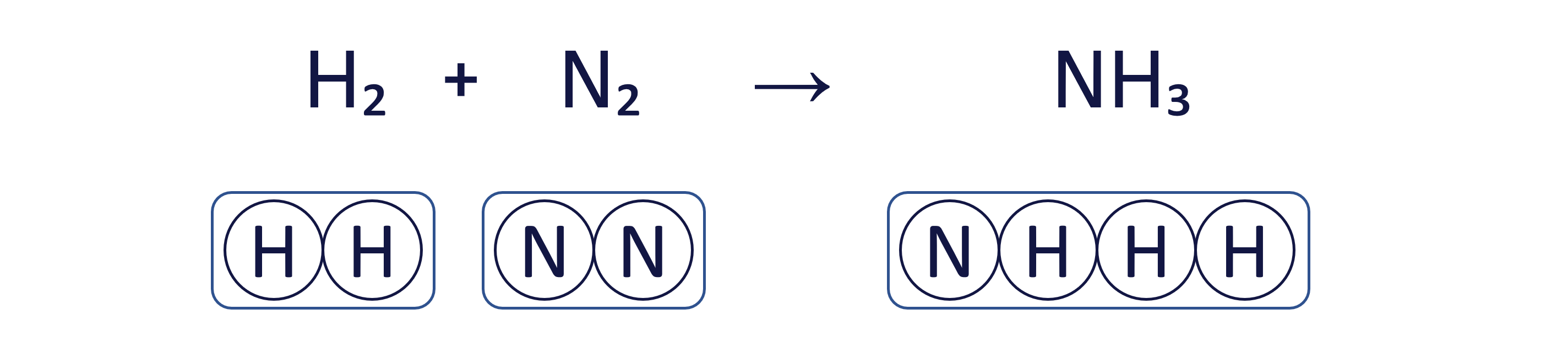
Worked Example - Balancing equations (intermediate)
To balance an equation, you must adjust the coefficients (the numbers placed before the chemical formulae) to ensure that the quantity of each element is the same on both sides.
The box method visually ensures that we do not change the formula of any substance, only the quantity of each 'box' (substance) to balance the equation.
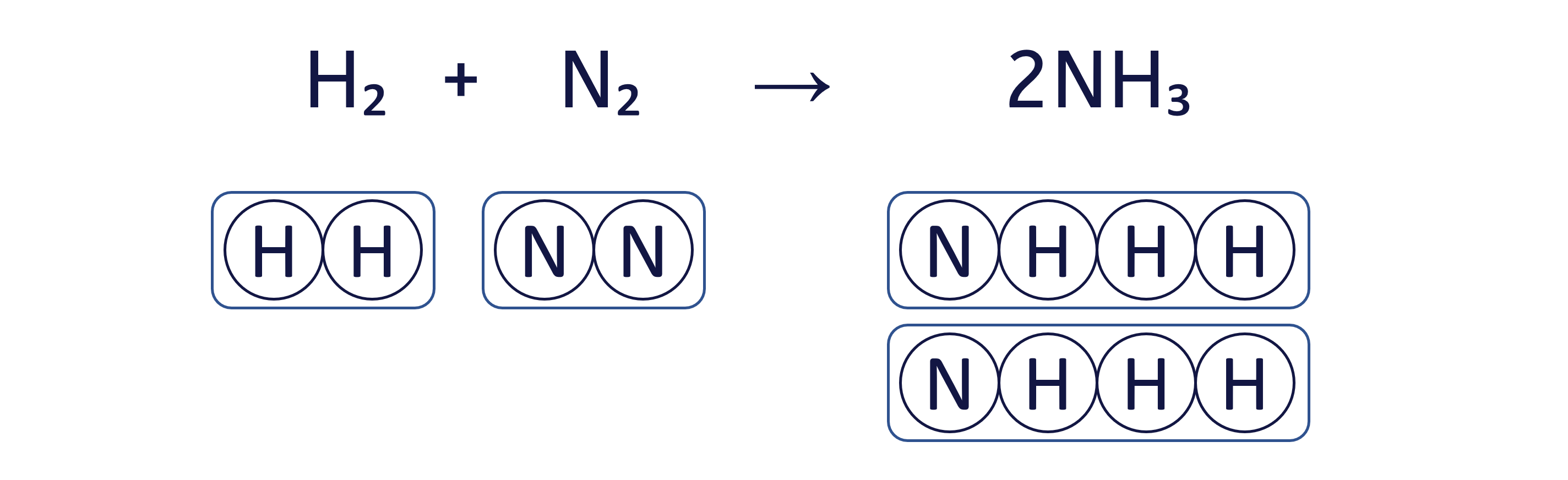
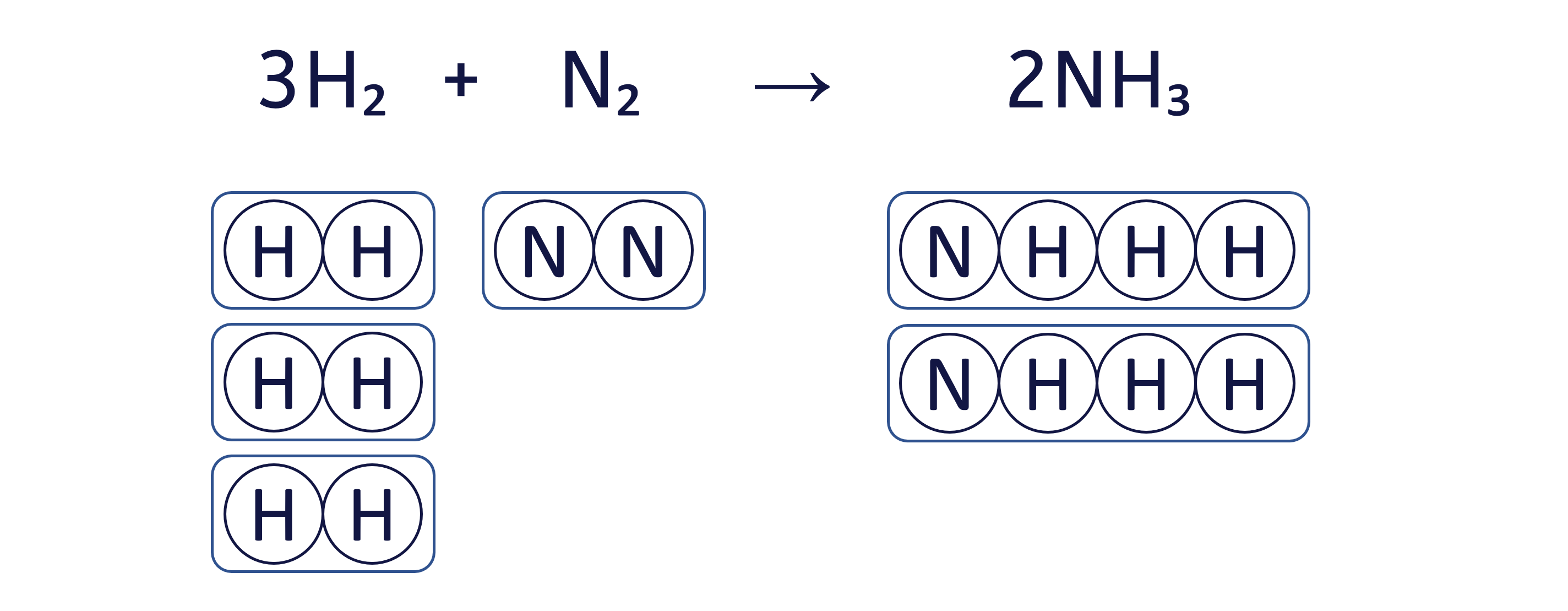
To balance the chemical equation H₂ + N₂ → NH₃ using the box method, follow these three steps:
Worked Example - Balancing equations (mastery)
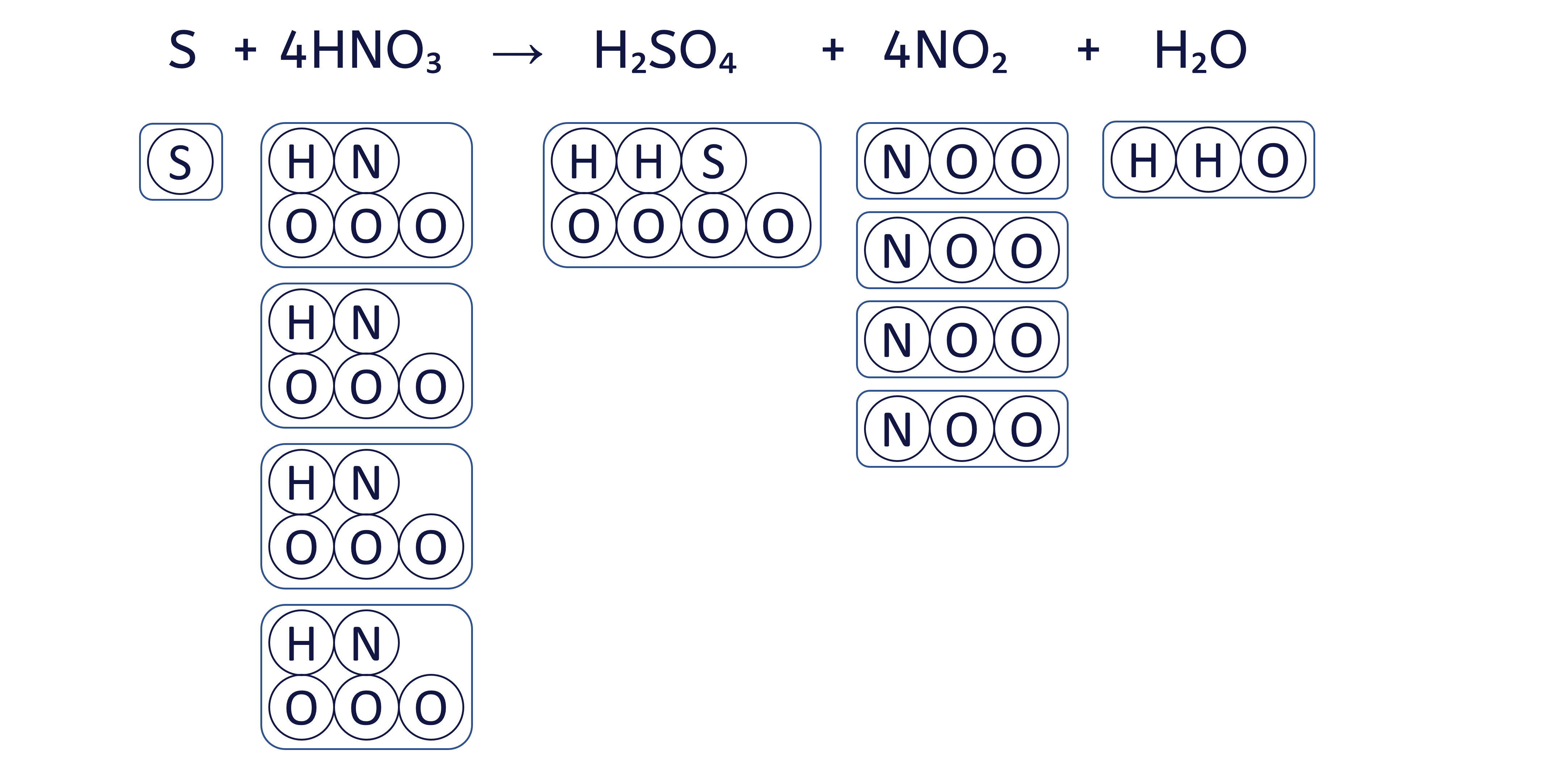
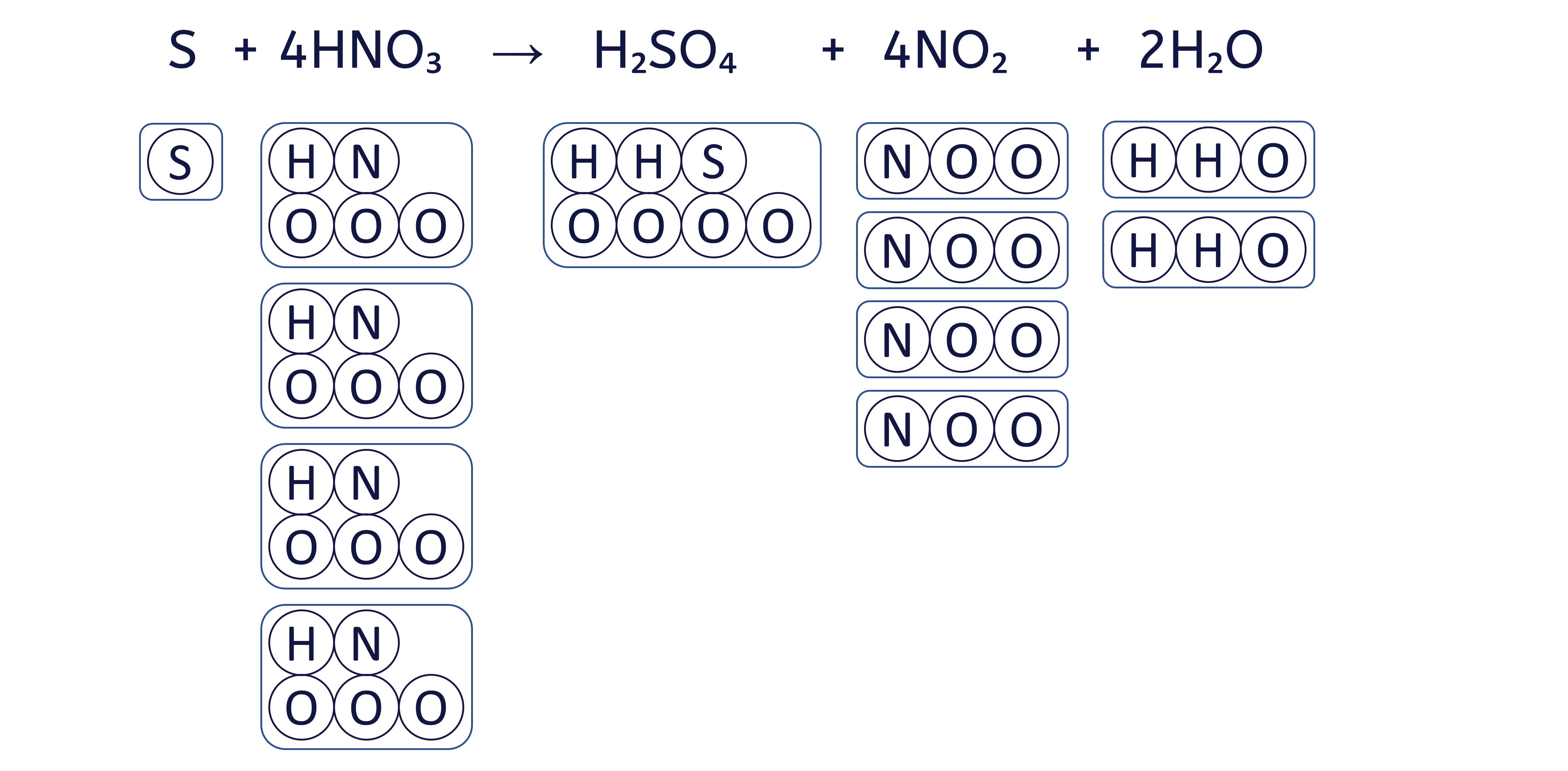
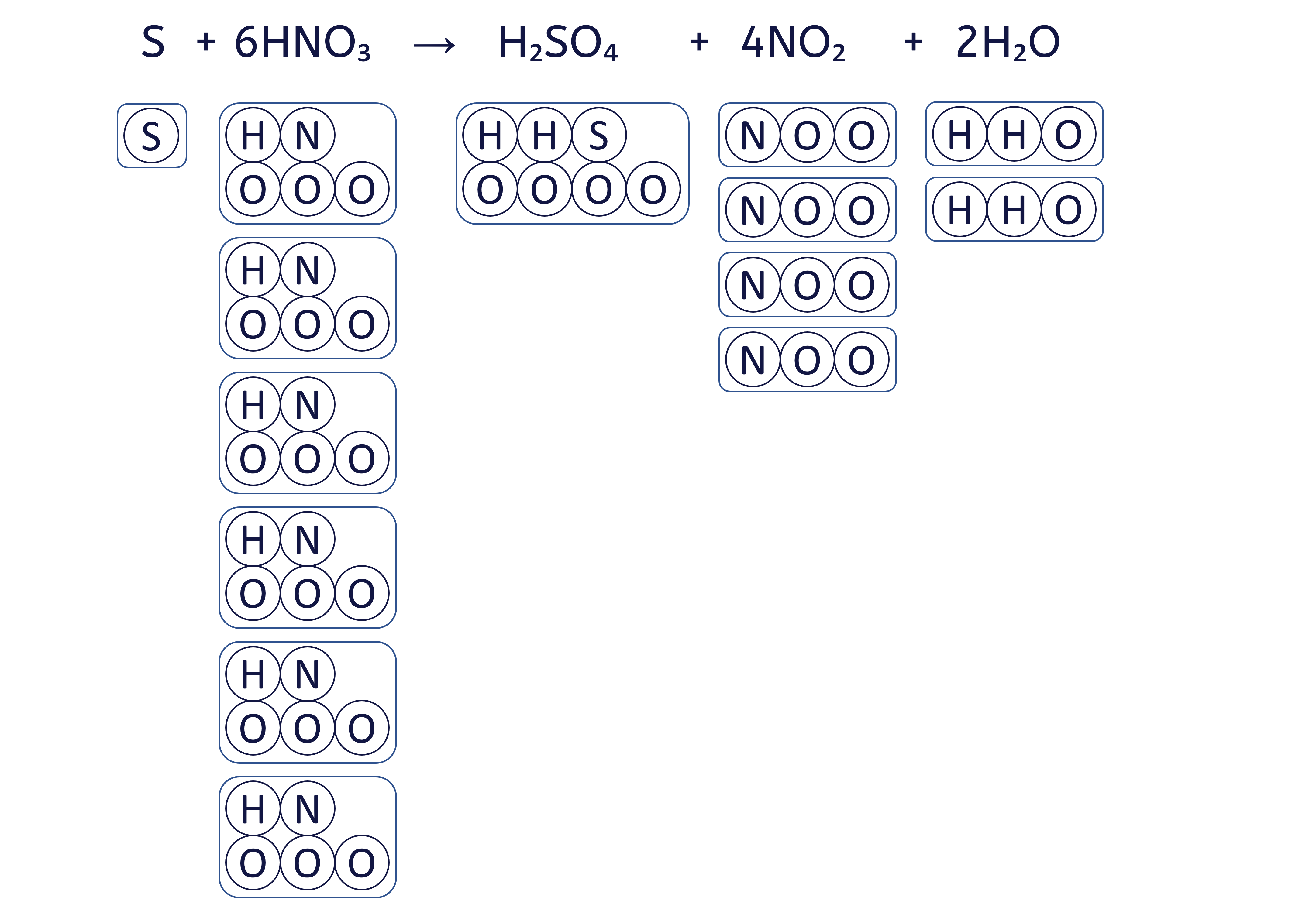
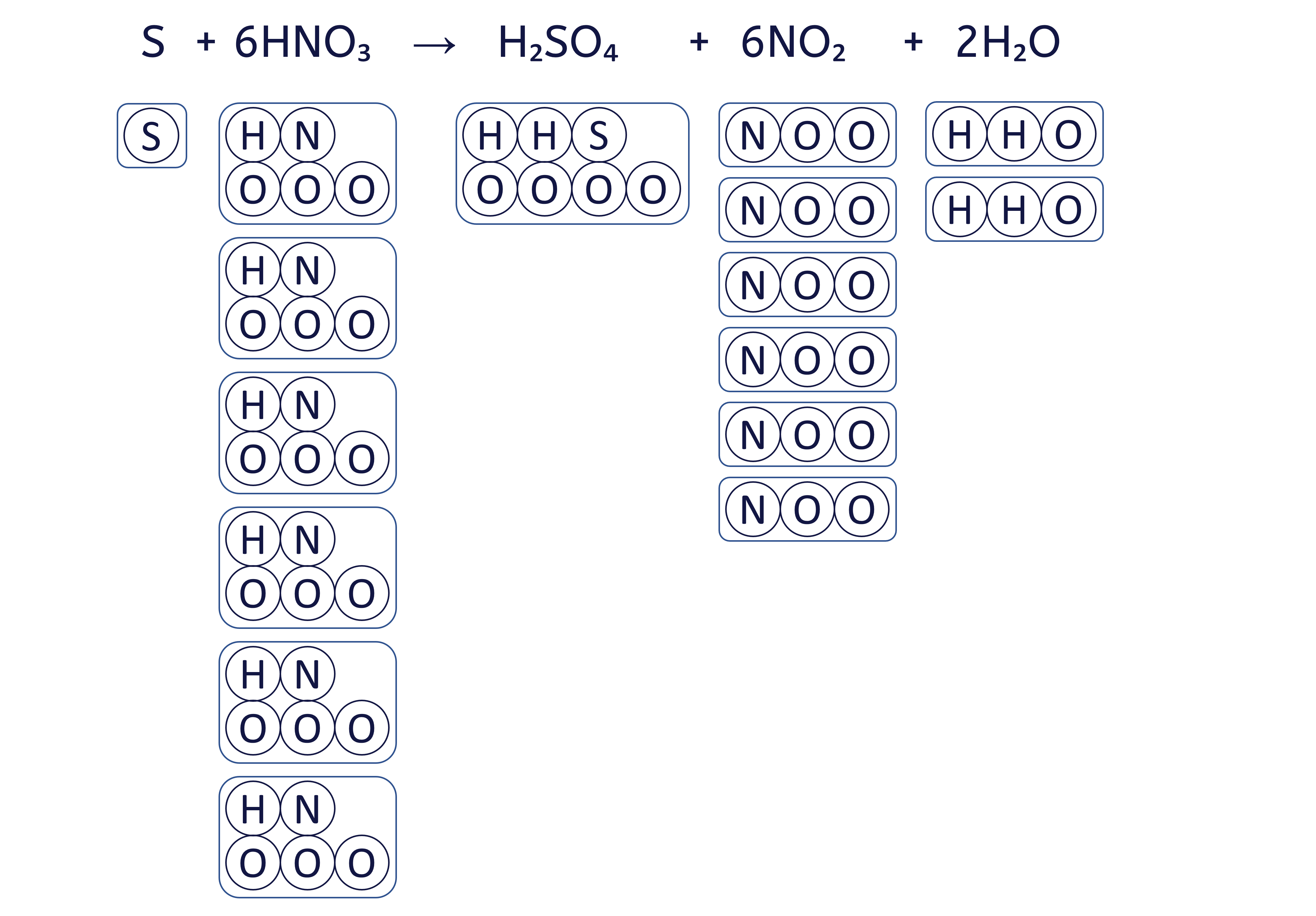
To balance an equation, you must adjust the coefficients (the numbers placed before the chemical formulae) to ensure that the quantity of each element is the same on both sides.
The box method visually ensures that we do not change the formula of any substance, only the quantity of each 'box' (substance) to balance the equation.
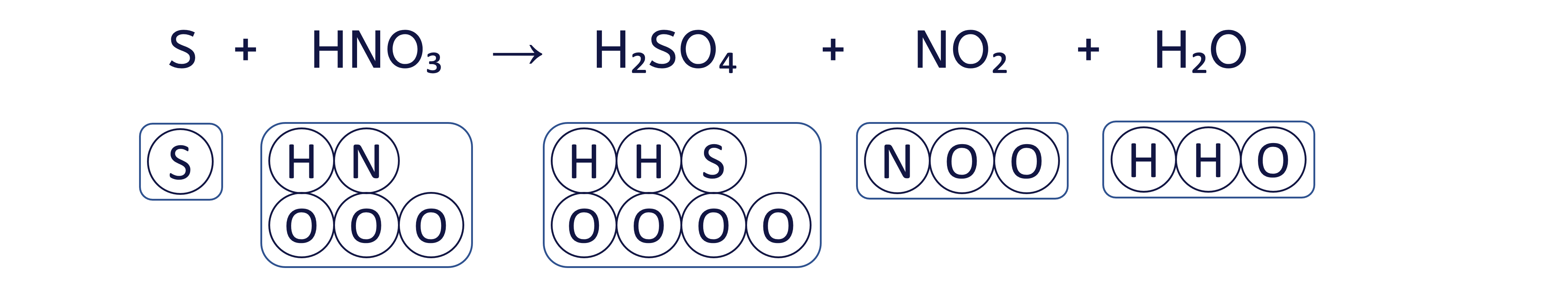
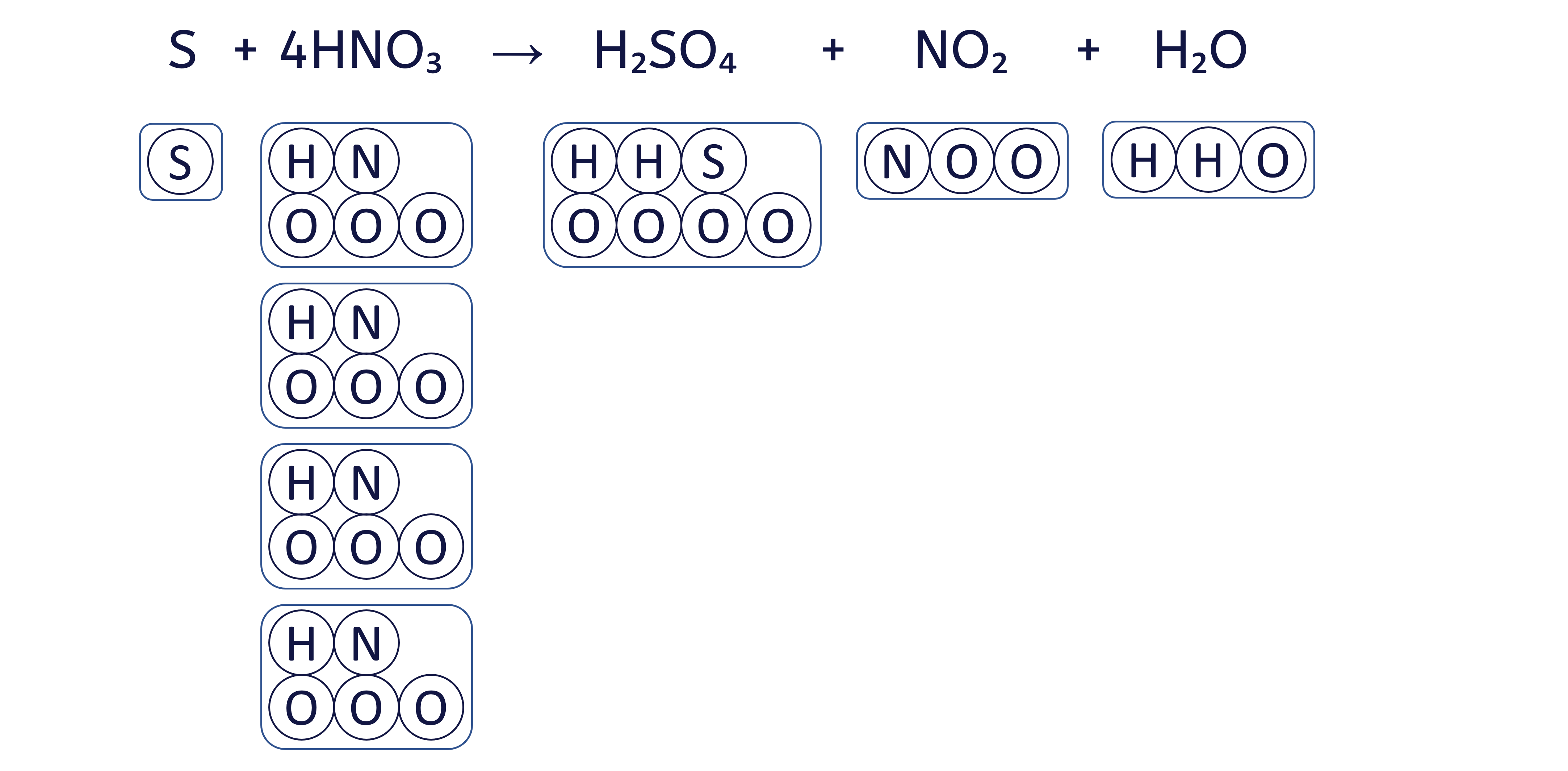
To balance the chemical equation S + HNO₃ → H₂SO₄ + NO₂ + H₂O using the box method, follow these six steps:
Ionic Equations
Ionic equations can be written from the overall reaction by simplifying the balanced symbol equation or by combining half equations. They provide a clear representation of the species involved in the reaction and how electrons are transferred.
To write an ionic equation from a balanced symbol equation, follow these steps:
- Identify and separate the aqueous ionic compounds into their constituent ions.
- Remove spectator ions (ions that appear unchanged on both sides of the equation).
- Write the remaining species to show the net ionic equation.
Worked Example - Ionic equation from balanced symbol equation
Write an ionic equation for the following reaction:
NaCl (aq) + AgNO₃ (aq) → NaNO₃ (aq) + AgCl (s)
Steps to write an ionic equation from a balanced symbol equation:
- Identify and separate the aqueous ionic compounds into their constituent ions.
- Na⁺ (aq) + Cl⁻ (aq) + Ag⁺ (aq) + NO₃⁻ (aq) → Na⁺ (aq) + NO₃⁻ (aq) + AgCl (s)
- AgCl remains unsplit as it is in solid form (the ions are not free to move)
- Remove spectator ions:
- Spectator ions exist in both reactants and products: Na⁺(aq) and NO₃⁻(aq)
- Na⁺ (aq) + Cl⁻ (aq) + Ag⁺ (aq) + NO₃⁻ (aq) → Na⁺ (aq) + NO₃⁻ (aq) + AgCl (s)
- Write the remaining species to show the net ionic equation:
- Cl⁻ (aq) + Ag⁺ (aq) → AgCl (s)
To write an ionic equation by combining half equations, follow these steps:
- Multiply the half equations by appropriate coefficients to balance the number of electrons in both equations.
- Add the half equations together, ensuring that the electrons cancel out.
- Simplify the equation to show only the species involved in the reaction.
Worked Example - Ionic equation from combining half equations
Combine the following half equations:
Fe (s) → Fe³⁺ (aq) + 3e⁻
Cl₂ (g) + 2e⁻ → 2Cl⁻ (aq)
Steps to write an ionic equation by combining half equations:
- Balance the numbers of electrons:
- Oxidation half equation: 2Fe (s) → 2Fe³⁺ (aq) + 6e⁻
- Reduction half equation: 3Cl₂ (g) + 6e⁻ → 6Cl⁻ (aq)
- Combine the equations:
- 2Fe (s) + 3Cl₂ (g) + 6e⁻ → 2Fe³⁺ (aq) + 6e⁻ + 6Cl⁻ (aq)
- Cancel out the electrons:
- 2Fe (s) + 3Cl₂ (g) → 2Fe³⁺ (aq) + 6Cl⁻ (aq)
Energy Changes in Reactions
Energy changes are a fundamental aspect of chemical reactions. They occur due to the breaking of old bonds and the formation of new ones, resulting in either the absorption or release of energy. Understanding these changes is crucial for predicting reaction behaviour and harnessing chemical reactions for practical purposes.
Every chemical reaction requires a certain amount of energy to get started, known as activation energy. This energy is needed to break the initial bonds in the reactants and start the reaction. Even exothermic reactions, which release energy overall, require an input of activation energy. However, the total amount of energy within an isolated system remains constant, as energy cannot be created or destroyed. This principle is known as the conservation of energy.
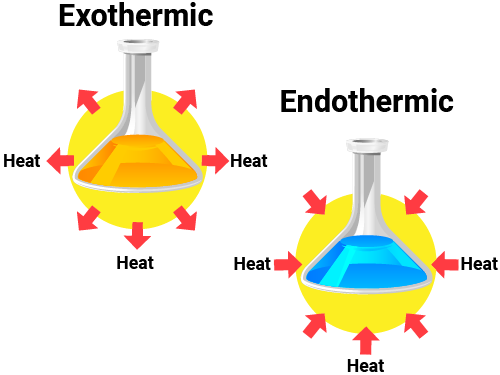
Chemical reactions can either absorb or release energy, leading to different types of reactions. The overall energy change in a reaction is the difference between the energy required to break the bonds in the reactants and the energy released when new bonds are formed in the products. An easy way to remember the difference between exothermic and endothermic reactions is to focus on the prefixes "exo-" and "endo-".
- Exothermic reactions: The prefix "exo-" means "outside". In these reactions, energy is released to the outside, or the surroundings. These reactions usually feel warm or hot because they give off heat. Examples include:
- combustion
- many oxidation reactions
- neutralisation
- displacements
- everyday examples: self-heating cans and handwarmers
- Endothermic reactions: The prefix "endo-" means "inside". These reactions absorb energy from the surroundings, leading to a decrease in temperature of the surroundings. These reactions usually feel cold because they take in heat. Examples include:
- electrolysis
- photosynthesis
- thermal decomposition
- reaction between citric acid and sodium hydrogencarbonate
- everyday example: some sports-injury packs
- Depending on the reactants, the following reactions can be either exothermic or endothermic:
- salts dissolving in water
- precipitation reactions
For example, when hydrogen reacts with oxygen to form water, the reaction releases energy, making it exothermic. In contrast, photosynthesis in plants is an endothermic reaction, as it requires energy from sunlight to convert carbon dioxide and water into glucose and oxygen.
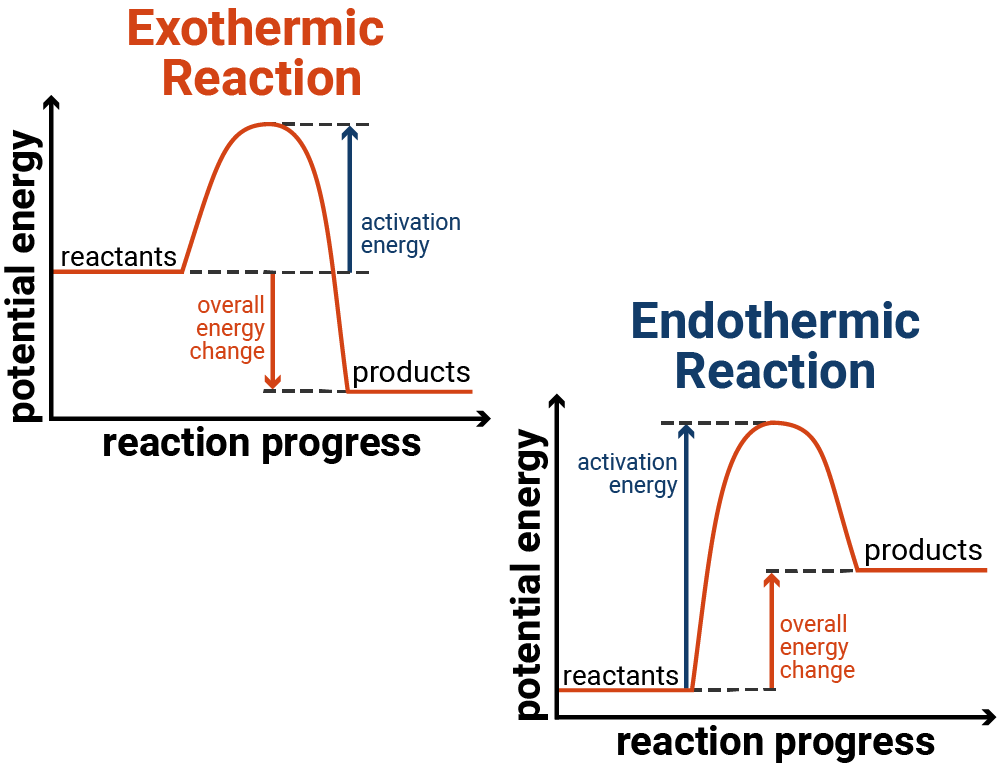
Reaction profiles, or energy diagrams, illustrate the energy changes during a chemical reaction. They show the energy of the reactants and products and the activation energy required to initiate the reaction:
- Exothermic reactions: In these reactions, the energy of the products is lower than that of the reactants. The reaction profile shows a peak representing the activation energy, followed by a drop in energy as the products form.
- Endothermic reactions: In these reactions, the energy of the products is higher than that of the reactants. The reaction profile shows a peak representing the activation energy, followed by a rise in energy as the products form.
Bond Energies
There are two parts to every chemical reaction:
- Bond breaking: This process requires energy to overcome the attractive forces holding atoms together. It is an endothermic process, meaning that energy is absorbed from the surroundings.
- Bond making: This process releases energy as new bonds form between atoms. It is an exothermic process, meaning that energy is released into the surroundings.
Consider the reaction between hydrogen (H₂) with oxygen (O₂) to form water (H₂O). The balanced equation is:
2H₂ + O₂ → 2H₂O
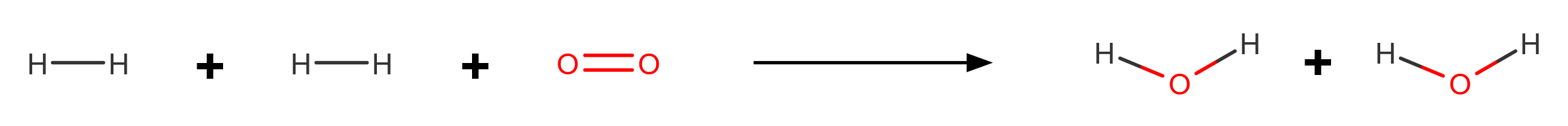
In this reaction, the H-H and O=O bonds in the reactants are broken, and new O-H bonds are formed in the products. The energy absorbed during bond breaking is less than the energy released during bond making, making the reaction exothermic overall.
In chemical reactions, the energy change can be calculated using the bond energies of the reactants and products. Bond energies are measured in kilojoules per mole (kJ/mol) and represent the amount of energy required to break one mole of a specific type of bond in a gaseous substance.
To work out the overall energy change in a reaction:
- Sum of bond energies of reactants: calculate the total energy required to break all the bonds in the reactants.
- Sum of bond energies of products: calculate the total energy released when the bonds in the products are formed.
- Net energy change = total energy required - total energy released
- If the energy change is negative this means it is an exothermic reaction, if it is positive then it is an endothermic reaction.
In discussing energy changes, we use positive (+) and negative (-) signs to indicate the direction of energy flow, not whether the energy itself is positive or negative.
- Positive energy change (+): This indicates that energy is absorbed by the reaction from the surroundings. It does not mean that the energy is positive, but rather that the reaction requires an input of energy. This is characteristic of endothermic reactions.
- Negative energy change (-): This indicates that energy is released by the reaction to the surroundings. It does not mean that the energy is negative, but rather that the reaction releases energy. This is characteristic of exothermic reactions.
Worked Example - Bond Energies
Consider the combustion of methane (CH₄) with oxygen (O₂) to form carbon dioxide (CO₂) and water (H₂O). The balanced equation is:
CH₄ + 2O₂ → CO₂ + 2H₂O
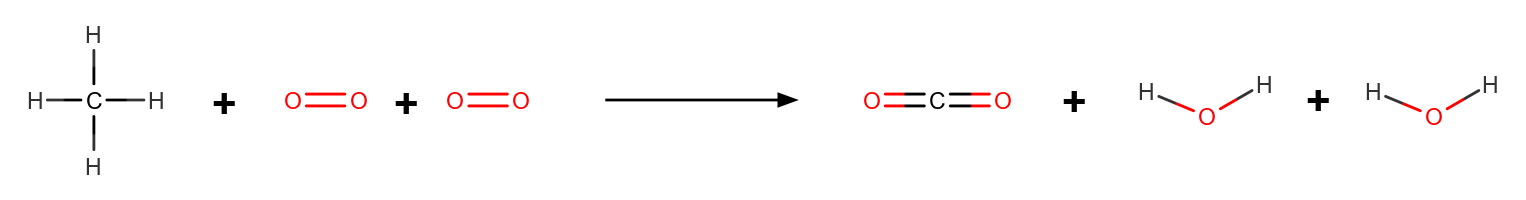
| bond | average bond energies (kJ/mol) |
|---|---|
| C-H | 412 |
| O=O | 498 |
| C=O | 799 |
| O-H | 463 |
Steps to calculate energy change:
- Sum of bond energies of reactants:
- Breaking 4 C-H bonds in CH₄: 4 × 412 kJ/mol = 1648 kJ/mol
- Breaking 2 O=O bonds in 2 O₂: 2 × 498 kJ/mol = 996 kJ/mol
- Total energy required: 1648 kJ/mol + 996 kJ/mol = 2644 kJ/mol
- Sum of bond energies of products:
- Forming 2 C=O bonds in CO₂: 2 × 799 kJ/mol = 1598 kJ/mol
- Forming 4 O-H bonds in 2 H₂O: 4 × 463 kJ/mol = 1852 kJ/mol
- Total energy released: 1598 kJ/mol + 1852 kJ/mol = 3450 kJ/mol
- Net energy change = total energy required - total energy released
- Net energy change = 2644 kJ/mol - 3450 kJ/mol = -806 kJ/mol
- The negative sign indicates that the reaction is exothermic, meaning it releases 806 kJ/mol of energy to the surroundings.
Listen to this page (feature coming soon)
Did you know?
- The vibrant colours in volcanic eruptions are due to various chemical reactions. For instance, the red hue is often from oxidised iron, while yellow can come from sulfur compounds.
- In the vacuum of space, chemical reactions can occur in the cold and dark, thanks to cosmic rays that provide the energy needed for reactions.
- Some modern technologies use chemical reactions accelerated by light, known as photocatalysis, to break down pollutants in water and air, making the environment cleaner.
Why do we care?
- Observing chemical reactions helps us understand everyday phenomena, such as rust forming on a bike left outside or the fizzing of vinegar and baking soda.
- Writing word and balanced symbol equations allows us to predict the outcomes of reactions, essential for tasks like baking (where ingredients react to form new products) and creating pharmaceuticals.
- Understanding ionic equations explains how substances like salts dissolve in water to conduct electricity, important for designing batteries and medical treatments.
- Learning about energy changes in reactions, such as exothermic reactions in hand warmers or endothermic reactions in instant cold packs, helps us use these products effectively.
Key information
- Reactants are converted into products through the breaking and forming of chemical bonds.
- Indicators of chemical reactions include energy changes (heat, light), colour changes, gas production, formation of solids, and changes in smell.
- Word equations: These describe the reactants and products of a chemical reaction in words, making it easier to understand the process.
- Balanced symbol equations: These provide a detailed representation of chemical reactions, showing the exact quantities of reactants and products while adhering to the law of conservation of mass. State symbols (s, l, g, aq) indicate the physical states of substances.
- Ionic equations: These can be derived by simplifying balanced symbol equations (removing spectator ions) or by combining half equations, providing a clear representation of the species involved and the electron transfers.
- Exothermic reactions release energy (heat) to the surroundings, and endothermic reactions absorb energy (heat) from the surroundings.
- Chemical reactions involve energy changes due to bond breaking (endothermic) and bond making (exothermic). The overall energy change can be calculated using bond energies.