
Acids, bases and pH (GCSE)
KS4 National Curriculum Statement(s) covered
- The chemistry of acids; reactions with some metals and carbonates
- pH as a measure of hydrogen ion concentration and its numerical scale
- Concentrations of solutions in relation to mass of solute and volume of solvent
- Identification of common gases
Skip to:
Acids and bases are essential substances in chemistry, affecting many aspects of our daily lives, from the food we eat to the cleaning products we use.
Antoine Lavoisier, a prominent French chemist, contributed significantly to our understanding of acids. He named oxygen (from Greek "oxys" meaning acid and "genes" meaning producer) because he mistakenly thought it was present in all acids. Though he was incorrect, the name stuck.
Acids and Bases
Acids are substances that release hydrogen ions (H⁺) when dissolved in water. They have a sour taste, can corrode metals, and are typically found in substances like lemon juice and vinegar.
| common acids | formula | example in everyday life | chemical name out of solution | formula |
|---|---|---|---|---|
| hydrochloric acid | HCl (aq) | found in gastric acid (in stomach) | hydrogen chloride | HCl (g) |
| sulfuric acid | H₂SO₄ (aq) | used in car batteries | sulfur trioxide | SO₃ (g) |
| nitric acid | HNO₃ (aq) | used in fertiliser production | nitrogen dioxide | NO₂ (g) |
| ethanoic acid | CH₃COOH (aq) | found in household vinegar | ethanoic acid | CH₃COOH (l) |
Bases are substances that can neutralise acids to form a salt and water only. Bases that are soluble in water are called alkalis, and they release hydroxide ions (OH⁻) when dissolved. Bases often have a bitter taste and a slippery feel, and they are found in substances like soap and baking soda.
| examples of bases | formula | example in everyday life |
|---|---|---|
| sodium hydroxide | NaOH | used in oven cleaners and soap making |
| calcium hydroxide (limewater) | Ca(OH)₂ | used in plaster (also used in chemistry labs as limewater) |
| ammonium hydroxide | NH₄OH (forms when dissolving ammonia, NH₃, in water) | used in household cleaning products |
| magnesium oxide | MgO | used in antacids to relieve indigestion |
Ammonium hydroxide forms when ammonia (NH₃) gas dissolves in water, producing a solution of ammonium ions (NH₄⁺) and hydroxide ions (OH⁻). The chemical reaction is:
- ammonia + water → ammonium hydroxide
- NH₃ (g) + H₂O (l) → NH₄OH (aq)
The strength of an acid or base depends on its ability to ionise in water. Strong acids and bases completely ionise in water, releasing more ions and therefore being more reactive. Examples include hydrochloric acid and sodium hydroxide. Weak acids and bases only partially ionise, making them less reactive, such as ethanoic acid.
Concentration, on the other hand, refers to the amount of solute present in a given volume of solution. A concentrated solution contains a large amount of solute, while a dilute solution contains a smaller amount. This distinction is important in both laboratory settings and industrial applications.
pH Scale and Indicators
The pH scale measures the acidity or alkalinity of a solution and ranges from 0 to 14. The pH of a solution can be measured precisely using a pH meter, which provides a numerical value. For less precise measurements, universal indicator (UI) paper or solution can be used. These chemical indicators change colour depending on the pH of the solution, providing a rough estimate of its acidity or alkalinity. It's important to note that UI paper and UI solution can produce slightly different colours at the same pH.
Lower pH values indicate higher concentrations of H⁺ ions (and lower concentrations of OH⁻ ions), while higher pH values indicate lower concentrations of H⁺ ions (and higher concentrations of OH⁻ ions).
| The pH scale | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ← increasing acidity | neutral | increasing alkalinity → | ||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| UI solution | ||||||||||||||
| UI paper | ||||||||||||||
The pH scale is a logarithmic scale based on the concentration of hydrogen ions (H⁺) in a solution, where each whole number change represents a tenfold change in H⁺ concentration. A pH of 7 is neutral, values below 7 are acidic, and values above 7 are alkaline.
This means:
- pH 1 has ten times more H⁺ ions than pH 2
- pH 11 has 100 times more OH⁻ ions than pH 9
- pH 6 has 1000 times fewer H⁺ ions than pH 3
It is important to note that pH does not have to be an integer - pH meters often provide readings to multiple decimal places. For determining if a substance is simply acidic (or not), such precision isn't necessary. Additionally, pH can also be a negative value, or have a value greater than 14, but these values are less common in typical laboratory and environmental conditions.
There are other chemical indicators that can determine whether a solution is acidic, neutral, or alkaline but these cannot measure the pH value:
| indicator | colour when in acidic solution | colour when in neutral solution | colour when in alkaline solution |
|---|---|---|---|
| red litmus paper | red | red | blue |
| blue litmus paper | red | blue | blue |
| litmus solution | red | purple | blue |
| methyl orange solution | red | yellow | yellow |
| universal indicator (UI) | red, orange or yellow | green | blue or purple |
| phenolphthalein solution | colourless | colourless | pink |
How universal indicator (UI) paper looks in different solutions:
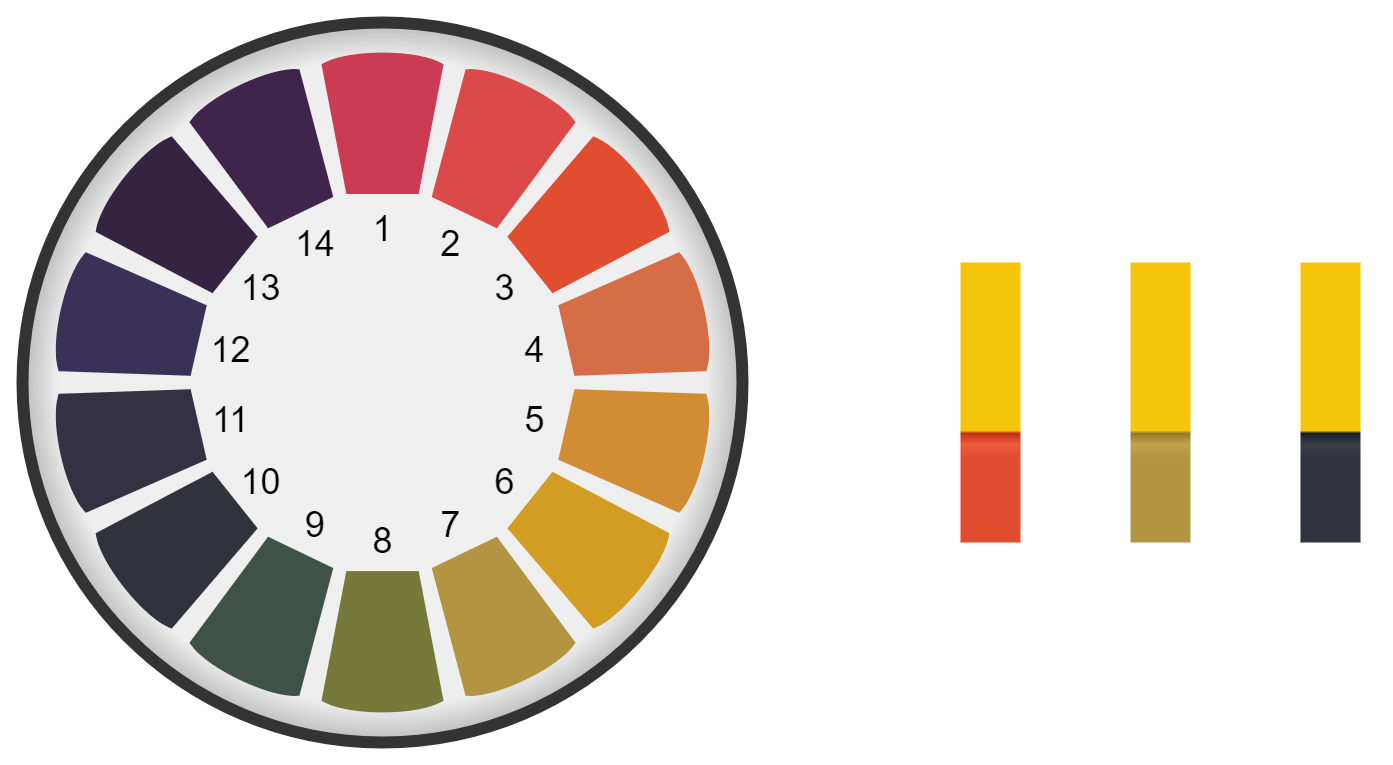
Where the indicator paper turns red (acidic), green (neutral) and blue (alkaline).
How litmus paper looks in different solutions:
.png?h=4254095044d8b63c3144fde26a69418e)
in acidic solutions:
blue litmus paper turns red, and
red litmus paper stays red
.png?h=4254095044d8b63c3144fde26a69418e)
in neutral solutions:
blue litmus paper stays blue, and
red litmus paper stays red
.png?h=4254095044d8b63c3144fde26a69418e)
in alkaline solutions:
blue litmus paper stays blue, and
red litmus turns blue
Reactions of Acids
A salt is a compound formed when the hydrogen ion (H⁺) in an acid is replaced by a metal ion or an ammonium ion (NH₄⁺). Salts are typically formed during neutralisation reactions between acids and bases. For example, when hydrochloric acid reacts with sodium hydroxide, sodium chloride (a salt) and water are produced.
When naming salts, the first part of the name comes from the positive ion (usually unchanged), and the second part of the name comes from the acid used (e.g. chloride, sulfate, nitrate, etc.). The table below shows some examples of the salts produced by different combinations of reactants and acids:
| copper oxide | sodium hydroxide | potassium carbonate | ammonia | |
|---|---|---|---|---|
| hydrochloric acid | copper chloride | sodium chloride | potassium chloride | ammonium chloride |
| sulfuric acid | copper sulfate | sodium sulfate | potassium sulfate | ammonium sulfate |
| nitric acid | copper nitrate | sodium nitrate | potassium nitrate | ammonium nitrate |
| ethanoic acid | copper ethanoate | sodium ethanoate | potassium ethanoate | ammonium ethanoate |
Acid + metal:
When acids react with certain metals, they produce a salt and hydrogen gas. This type of reaction is not only a simple chemical reaction but also an example of a redox (reduction-oxidation) reaction.
The general equation is:
acid + metal → salt + hydrogen
Example with hydrochloric acid (HCl (aq)) and sodium (Na):
- hydrochloric acid + sodium → sodium chloride + hydrogen
- 2HCl (aq) + 2Na (s) → 2NaCl (aq) + H₂ (g)
In a redox reaction, oxidation and reduction occur simultaneously. Oxidation refers to the loss of electrons, while reduction refers to the gain of electrons. Here’s how this applies to the reaction between hydrochloric acid and sodium:
- Oxidation: Each sodium atom loses one electron to form a sodium ion.
Na (s) → Na⁺ (aq) + e⁻
Sodium (Na) is oxidised because it loses electrons. - Reduction: Hydrogen ions from the hydrochloric acid gain the released electrons to form hydrogen gas.
2H⁺ (aq) + 2e⁻ → H₂ (g)
Hydrogen ions (H⁺) are reduced because they gain electrons.
Hydrogen gas can be tested for by bringing a lit splint near a container of the gas. If a 'squeaky pop' is heard, then hydrogen gas is present.
Acid + base:
Acids react with bases to produce a salt and water. The general equation is:
acid + base → salt + water
acid + alkali → salt + water
This reaction is called a neutralisation reaction.
Example with sulfuric acid (H₂SO₄ (aq)) and sodium hydroxide (NaOH):
- sulfuric acid + sodium hydroxide → sodium sulfate + water
- H₂SO₄ (aq) + 2NaOH (aq) → Na₂SO₄ (aq) + 2H₂O (l)
Special case of ammonia (NH₃)
Ammonia is a basic gas. This means that it dissolves, and reacts, in water to form an alkaline solution (containing ammonium hydroxide). We can write chemical equations where substances react with ammonia, however when water is present (like in acidic solutions) the reaction is actually with ammonium hydroxide.
Hydrochloric acid (HCl (aq)) and ammonia (NH₃ (g)) react to form only one product:
- hydrochloric acid + ammonia → ammonium chloride
- HCl (aq) + NH₃ (g) → NH₄Cl (aq)
Hydrochloric acid (HCl (aq)) and ammonium hydroxide (NH₄OH (aq)) react to form two products:
- hydrochloric acid + ammonium hydroxide → ammonium chloride + water
- HCl (aq) + NH₄OH (aq) → NH₄Cl (aq) + H₂O (l)
Neutralisation reactions involve acids reacting with bases to form a salt and water. When we write the equation only reacting with ammonia this doesn't appear to produce any water (producing only a salt). However, in reality the acid is actually reacting with ammonium hydroxide, thus producing a salt and water.
Acid + metal carbonate:
Acids react with metal carbonates to produce a salt, water, and carbon dioxide gas. The general equation is:
acid + metal carbonate → salt + water + carbon dioxide
At GCSE you need to know that bases are substances that neutralise acids to form a salt and water only. Metal carbonates don't strictly meet this definition as they also produce carbon dioxide. However, generally metal carbonates are considered to be bases.
Example with nitric acid (HNO₃ (aq)) and sodium carbonate (Na):
- nitric acid + sodium carbonate → sodium nitrate + water + carbon dioxide
- 2HNO₃ (aq) + Na₂CO₃ (aq) → 2NaNO₃ (aq) + H₂O (l) + CO₂ (g)
Carbon dioxide gas can be tested for by bubbling it through a solution of limewater. If the limewater turns cloudy (milky), then carbon dioxide is present.
Deducing the Formulae of Salts
Understanding how to name salts and determine their chemical formulae is a fundamental skill in chemistry. Salts are ionic compounds, and form through reactions with many substances (e.g. when an acid reacts with a base, metal, or carbonate).
To deduce the formula of a salt, you need to know the charges of the ions involved. Salts are composed of positive ions (cations) and negative ions (anions) that combine in such a way that the overall charge is zero:
- Identify the cation and anion:
- The cation is typically a metal ion or ammonium ion (NH₄⁺).
- The anion is derived from the acid.
- Use the periodic table or a list of common ions to determine the charges of the cation and anion.
- Combine the cation and anion in a ratio that balances the overall charge.
- Write the formula.
| monatomic ion | formulae | polyatomic ion | formula |
|---|---|---|---|
| Group 1 metals | e.g. Li⁺, Na⁺, K⁺ | hydroxide | OH⁻ |
| Group 2 metals | e.g. Mg²⁺, Ca²⁺ | nitrate | NO₃⁻ |
| Group 3 metals | e.g. Al³⁺ | sulfate | SO₄²⁻ |
| Group 5 non-metals | e.g. N³⁻ | carbonate | CO₃²⁻ |
| Group 6 non-metals | e.g. O²⁻, S²⁻ | phosphate | PO₄³⁻ |
| Group 7 non-metals | e.g. Cl⁻, Br⁻, I⁻ | ammonium | NH₄⁺ |
Worked Example - Deducing salt formulae, sodium chloride
- Identify the cation and anion:
- Sodium (Na) is in Group 1 of the periodic table and loses one electron to form Na⁺.
- Chlorine (Cl) is in Group 7 and gains one electron to form Cl⁻.
- Combine the cation and anion in a ratio that balances the overall charge.
- The charges on the sodium and chloride ions are +1 and -1, respectively.
- Since the charges are equal and opposite, they combine in a 1:1 ratio to neutralise each other.
- Write the formula
- Combine one Na⁺ ion with one Cl⁻ ion.
- The formula is NaCl.
Na⁺ + Cl⁻ → NaCl
Worked Example - Deducing salt formulae, calcium nitrate
- Identify the cation and anion:
- Calcium (Ca) is in Group 2 and loses two electrons to form Ca²⁺.
- The nitrate ion (NO₃⁻) is a polyatomic ion with a charge of -1.
- Combine the cation and anion in a ratio that balances the overall charge.
- The calcium ion has a charge of +2, and each nitrate ion has a charge of -1.
- To balance the charges, you need two nitrate ions to neutralise one calcium ion.
- Write the formula
- Combine one Ca²⁺ ion with two NO₃⁻ ions.
- The formula is Ca(NO₃)₂.
Ca²⁺ + 2NO₃⁻ → Ca(NO₃)₂
The brackets in Ca(NO₃)₂ indicate that the nitrate group (NO₃) is a polyatomic ion, and two of these groups are needed to balance the charge of one Ca²⁺ ion.
Worked Example - Deducing salt formulae, aluminium sulfate
- Identify the cation and anion:
- Aluminium (Al) is in Group 3 and loses three electrons to form Al³⁺.
- The sulfate ion (SO₄²⁻) is a polyatomic ion with a charge of -2.
- Combine the cation and anion in a ratio that balances the overall charge.
- The charge on Al³⁺ is +3, and the charge on SO₄²⁻ is -2.
- To balance the total charge, you need two Al³⁺ ions (2 × +3 = +6)
and three SO₄²⁻ ions (3 × -2 = -6)
- Write the formula
- Combine one Ca²⁺ ion with two NO₃⁻ ions.
- Two Al³⁺ ions combine with three SO₄²⁻ ions to form Al₂(SO₄)₃.
2Al³⁺ + 3SO₄²⁻ → Al₂(SO₄)₃
The brackets in Al₂(SO₄)₃ indicate that the sulfate group (SO₄) is a polyatomic ion, and three of these groups are needed to balance the charge of two Al³⁺ ions.
Concentration and Titrations
Concentration refers to the amount of solute dissolved in a given volume of solution and can be expressed in grams per cubic decimetre (g/dm³) or moles per cubic decimetre (mol/dm³). The concentration in g/dm³ is calculated by dividing the mass of the solute by the volume of the solution:
Grams per cubic decimetre (g/dm³):
- Measures the mass of solute per volume of solution.
- \( \text{concentration (g/dm³)} = \left(\frac{\text{mass of solute (g)}}{\text{volume of solvent (dm³)}}\right) \)
- Example: Dissolving 10 grams of salt in 1 dm³ of water gives a concentration of 10 g/dm³.
Moles per cubic decimetre (mol/dm³)
- Measures the amount of substance (in moles) per volume of solution.
- \( \text{concentration (mol/dm³)} = \left(\frac{\text{amount of substance (mol))}}{\text{volume of solvent (dm³)}}\right) \)
- Example: Dissolving 1 mole of salt in 1 dm³ of water gives a concentration of 1 mol/dm³.
Warning: External video
Titrations are used to determine an unknown concentration of a solution (the titrand) by reacting it with a solution of known concentration (titrant). A chemical indicator shows the end point of the reaction.
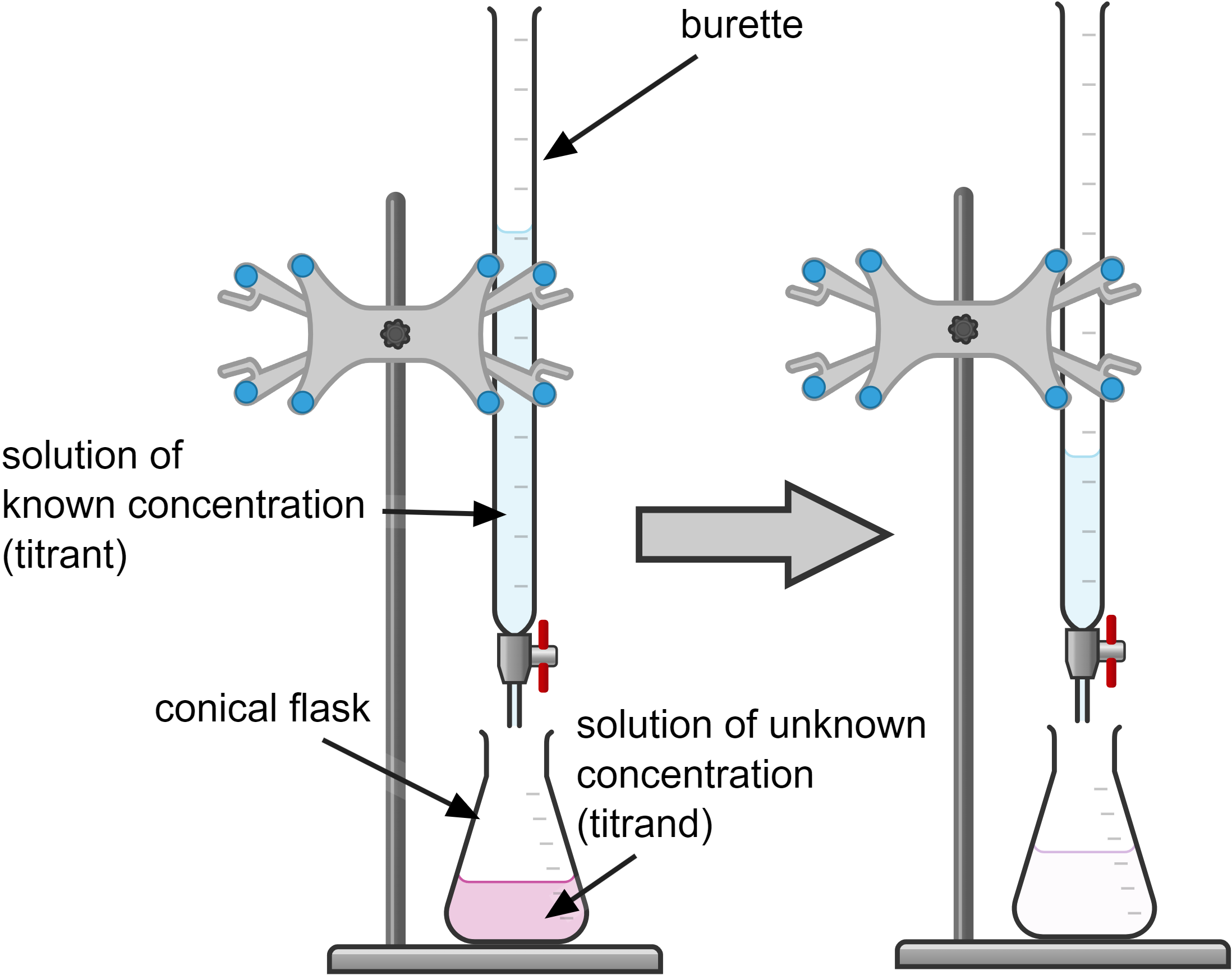
Here is a step-by-step guide to performing a titration to find an unknown concentration:
- Use the pipette and pipette filler to add a measured volume of the alkali with an unknown concentration (titrand) to a conical flask.
- Add a few drops of a suitable chemical indicator.
- Fill the burette with a solution with known concentration of the acid (titrant) and record the starting volume.
- Slowly add the acid from the burette to the alkali while swirling the flask until the chemical indicator changes colour, indicating the endpoint.
- Record the final volume of the acid.
- Repeat the titration to get concordant results (titres within 0.10 cm³ of each other).
- Use the concordant titres to calculate the mean titre.
- Check the balanced symbol equation. Calculate the concentration of the unknown solution using the formula: C₁V₁ = C₂V₂
Tips for accurate titration
- Continually swirl the flask to ensure complete mixing of acid and alkali.
- Wash the inside of the conical flask with a little distilled water to wash reactants on the sides into the reaction mixture.
- Wash the burette and pipette with the appropriate solution before titration to ensure they are not contaminated.
- Read the bottom of the meniscus at eye level to obtain accurate volume readings.
- Add the solution drop by drop near the endpoint to identify exactly when the chemical indicator changes colour.
- Use a white tile under the conical flask to make it easier to see the colour change.
- Ensure there are no air bubbles in the burette or pipette when measuring volumes to record exact volumes.
Common mistakes to avoid:
- Contaminated pipettes or burettes can introduce errors. Always rinse with the solution you will be using.
- Near the endpoint, add the titrant drop by drop to avoid overshooting the endpoint.
- Use an appropriate chemical indicator for the titration. Universal indicator is not suitable as it does not provide a sharp colour change at the endpoint.
Worked Example - Titration and calculation (1:1 ratio)
In this example, we'll determine the concentration of a sodium hydroxide (NaOH) solution using titration with a solution of hydrochloric acid (HCl) with known concentration of 0.10 mol/dm³.
The balanced chemical equation for the reaction between NaOH and HCl is:
NaOH + HCl → NaCl + H₂O
- Use a pipette and pipette filler to transfer 25.0 cm³ of the NaOH solution into a clean conical flask.
- Add a few drops of phenolphthalein indicator to the NaOH solution in the conical flask. The solution will turn pink, indicating it is basic.
- Rinse the burette with the HCl solution and fill it with the same solution. Record the initial volume of HCl in the burette.
- Slowly add the HCl from the burette to the NaOH solution, swirling the flask continuously.
- As you approach the endpoint (when the pink colour starts to fade), add the HCl drop by drop until the pink colour just disappears, indicating that neutralisation has occurred. Record the final volume of HCl in the burette.
- Repeat the titration until you obtain concordant results (titres within 0.10 cm³ of each other).
| run | final volume (cm³) | initial volume (cm³) | titre (cm³) |
|---|---|---|---|
| rough | 28.35 | 0.00 | 26.35 |
| 1 | 26.50 | 0.00 | 26.50 |
| 2 | 26.55 | 0.15 | 26.40 |
| 3 | 26.50 | 0.05 | 26.45 |
Runs 1-3 are all concordant titres (within 0.10 cm³), so can be used to calculate the mean titre.
The mean titre = 24.50 cm³
From the equation, we see that the reaction ratio between NaOH and HCl is 1:1. Therefore: C₁V₁ = C₂V₂
where:
- C₁ = concentration of NaOH (unknown)
- V₁ = volume of NaOH solution used (25.0 cm³)
- C₂ = concentration of HCl (0.10 mol/dm³)
- V₂ = mean titre of HCl (26.45 cm³)
First, convert the volumes from cm³ to dm³ (1000 cm³ = 1 dm³):
- V₁ = volume of NaOH solution used (0.025 dm³)
- V₂ = mean titre of HCl (0.02645 cm³)
Using the titration formula:
- C₁V₁ = C₂V₂
- C₁ × 0.025 = 0.10 × 0.02645
- C₁ × 0.025 = 0.002645
- \( \text{C₁} = \left(\frac{\text{0.002645}}{\text{0.025}}\right) \)
- C₁ = 0.1058 mol/dm³
The concentration of the sodium hydroxide solution is 0.1058 mol/dm³.
We need to give this to an appropriate number of significant figures (s.f.), usually this is to the lowest number of significant figures used (i.e. the concentration of hydrochloric acid used is quoted as 0.10 mol/dm³, which is only 2 s.f.).
The concentration of the sodium hydroxide solution is therefore 0.11 mol/dm³ (2 s.f.).
Worked Example - Titration calculation (2:1 ratio)
When using sulfuric acid (H₂SO₄) instead of hydrochloric acid (HCl), the stoichiometry of the reaction must be taken into account because the reaction ratio is different. Sulfuric acid provides two hydrogen ions (H⁺) per molecule, so the reaction ratio between NaOH and H₂SO₄ is 2:1.
We'll determine the concentration of a sodium hydroxide (NaOH) solution using titration with a solution of sulfuric acid (H₂SO₄) with known concentration of 0.10 mol/dm³.
The balanced chemical equation for the reaction between NaOH and HCl is:
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
This equation shows that two moles of sodium hydroxide react with one mole of sulfuric acid.
| run | final volume (cm³) | initial volume (cm³) | titre (cm³) |
|---|---|---|---|
| rough | 14.55 | 0.00 | 14.55 |
| 1 | 13.20 | 0.00 | 13.20 |
| 2 | 14.60 | 1.30 | 13.30 |
| 3 | 13.25 | 0.05 | 13.20 |
Runs 1-3 are all concordant titres (within 0.10 cm³), so can be used to calculate the mean titre.
The mean titre = 13.23 cm³
Since the reaction ratio is 2:1, we need to take this into account in our calculations.
First, convert the volumes from cm³ to dm³ (1000 cm³ = 1 dm³):
- C₁ = concentration of NaOH (unknown)
- V₁ = volume of NaOH solution used (0.025 dm³)
- C₂ = concentration of H₂SO₄ (0.10 mol/dm³)
- V₂ = mean titre of H₂SO₄ (0.01323 dm³)
Next, find the moles of H₂SO₄ used:
n(H₂SO₄) = C₂ × V₂ = 0.10 mol/dm³ × 0.01323 dm³ = 0.001323 mol
Since the ratio of NaOH to H₂SO₄ is 2:1:
n(NaOH) = 2 × n(H₂SO₄)
n(NaOH) = 2 × 0.001323 mol = 0.002646 mol
Now, use the volume of NaOH solution to find its concentration:
- \( \text{C₁} = \left(\frac{\text{n(NaOH)}}{\text{V₁}}\right) \)
- \( \text{C₁} = \left(\frac{\text{0.002646 mol}}{\text{0.025 dm³}}\right) \)
- C₁ = 0.10584 mol/dm³
The concentration of the sodium hydroxide solution is 0.10584 mol/dm³.
We need to give this to an appropriate number of significant figures (s.f.), usually this is to the lowest number of significant figures used (i.e. the concentration of hydrochloric acid used is quoted as 0.10 mol/dm³, which is only 2 s.f.).
The concentration of the sodium hydroxide solution is therefore 0.11 mol/dm³ (2 s.f.).
Making Salts
Understanding solubility rules helps determine which salts are soluble or insoluble in water:
| soluble compounds | insoluble compounds |
|---|---|
| all common sodium, potassium, and ammonium salts | - |
| all nitrates | - |
| most common chlorides | silver chloride, lead chloride |
| most common sulfates | lead sulfate, barium sulfate, calcium sulfate |
| sodium, potassium, and ammonium carbonates | most carbonates |
| sodium, potassium, and ammonium hydroxides | most hydroxides |
Making pure samples of soluble salts (with one insoluble reactant)
To make a soluble salt from an insoluble substance, we react an acid with an insoluble reactant. The insoluble reactant can potentially be a metal, a metal oxide, a metal hydroxide, or a carbonate. Here's how the process works:
- Choose the reactants:
- The acid is chosen based on the desired salt.
- The insoluble reactant is chosen based on its ability to react safely with the acid.
- Add powdered insoluble reactant to the acid in a beaker, one spatula at a time, stirring to mix.
- You may need to gently heat this mixture to increase the rate of reaction.
- Continue adding powder until some unreacted powder is left over, indicating that all the acid has reacted.
- Filter the mixture to remove the excess solid. The filtrate contains the soluble salt and water.
- Heat the solution in an evaporating dish over a water bath until small crystals start to appear around the edge of the dish. This indicates it is a saturated solution.
- Let the saturated solution to cool down to room temperature, and leave for a day or two to allow large crystals to form.
- If necessary, dry the crystals by gently dabbing with filter paper (or in a warm oven).
Worked Example - Making copper sulfate
We want to produce copper sulfate, a soluble salt, using sulfuric acid. Copper metal itself is not suitable for this reaction (it is not reactive enough), but we can use copper oxide (CuO), copper hydroxide (Cu(OH)₂), or copper carbonate (CuCO₃).
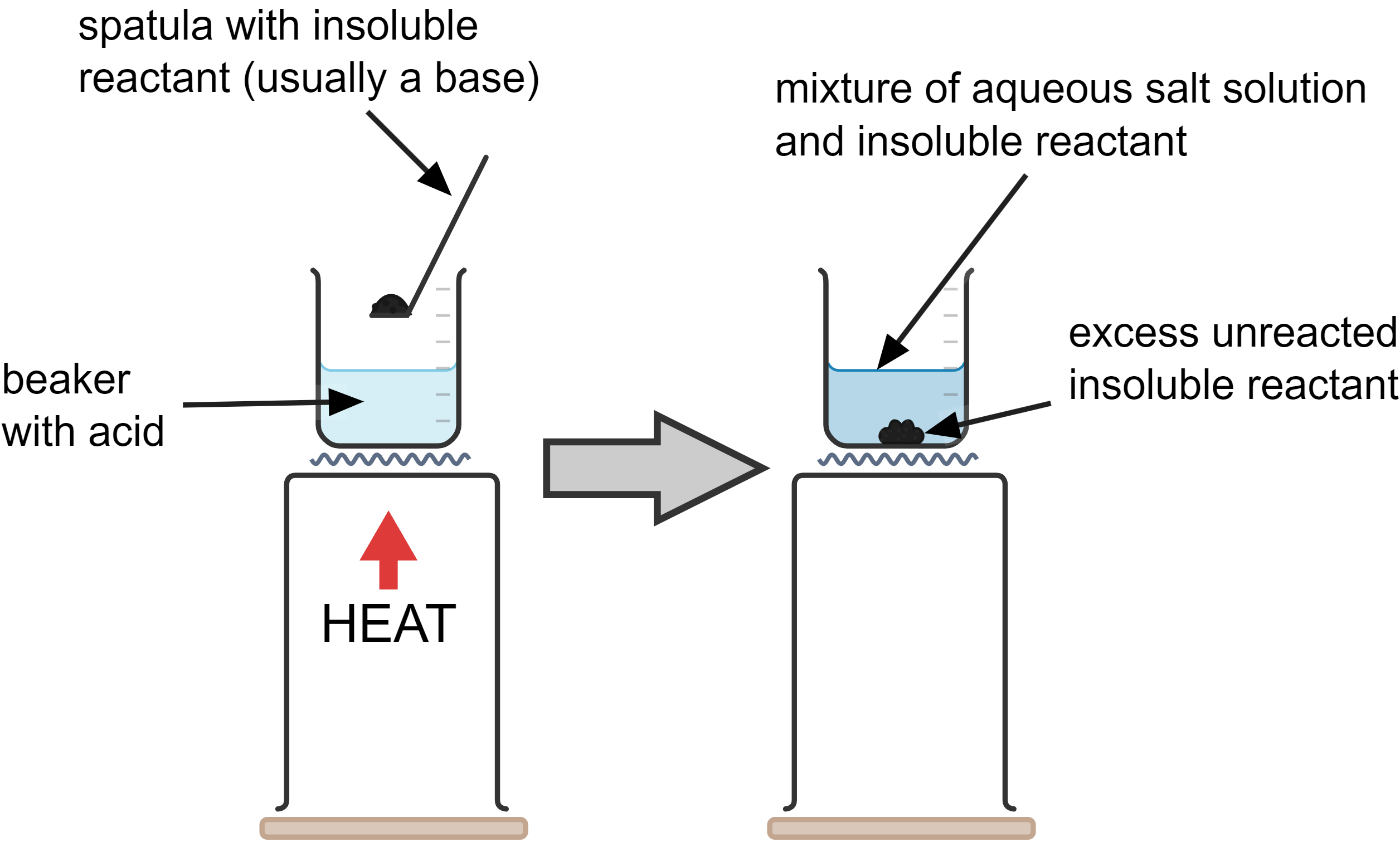
- Choose the reactants:
- Acid: Sulfuric acid (H₂SO₄) is chosen because we want to produce copper sulfate.
- Insoluble Reactant: For this example, we will use copper oxide (CuO).
- Add powdered copper oxide (CuO) to the sulfuric acid in a beaker, one spatula at a time, stirring to mix.
- Gently heat this mixture to increase the rate of reaction.
- Continue adding the copper oxide until some unreacted powder is left over. This indicates that all the acid has reacted.
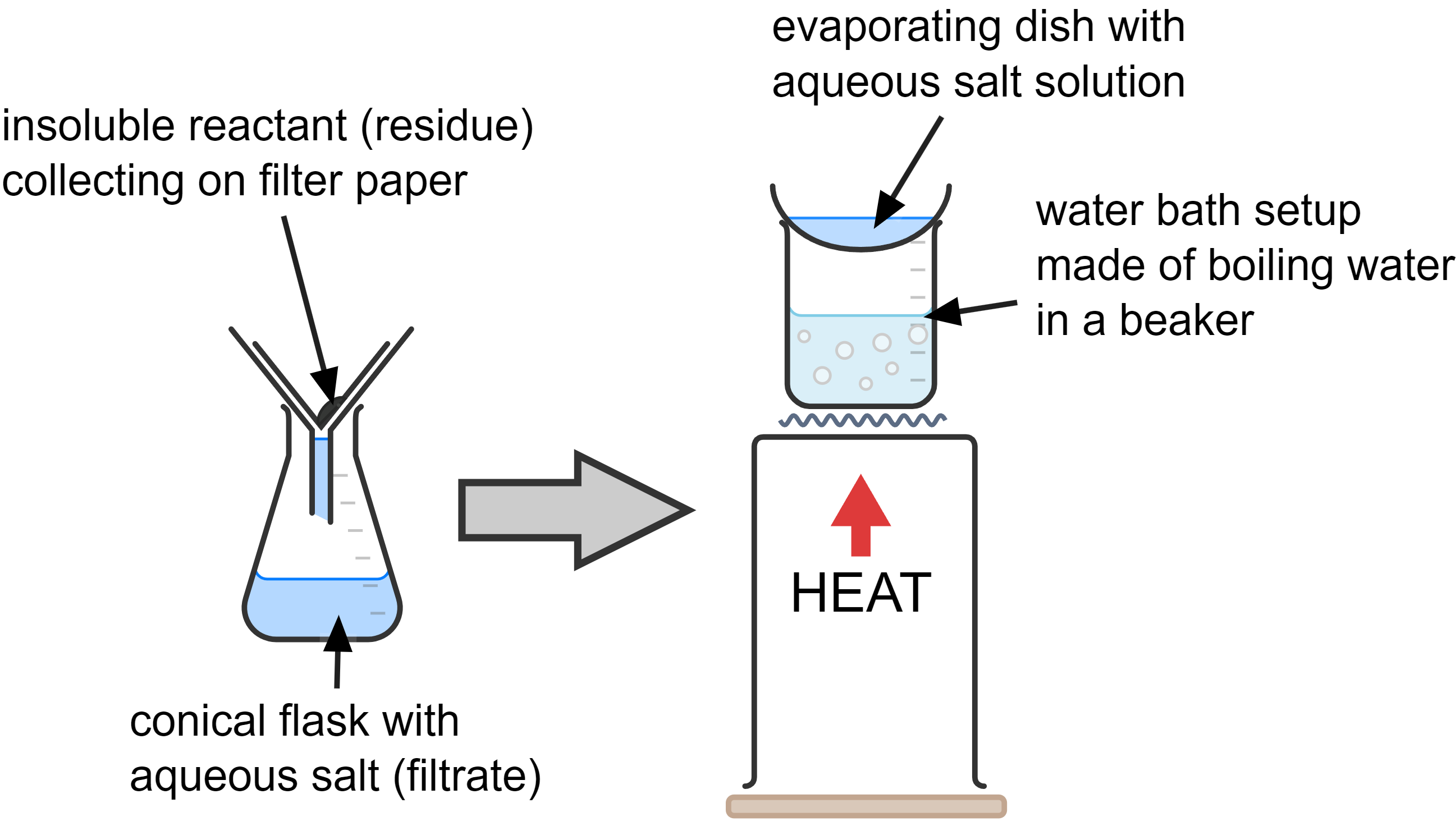
- Filter the mixture to remove the excess solid copper oxide. The filtrate contains the soluble copper sulfate and water.
- Heat the solution in an evaporating dish over a water bath until small crystals start to appear around the edge of the dish. This indicates it is a saturated solution.
- Let the saturated solution cool down to room temperature and leave it for a day or two to allow large crystals to form.
- If necessary, dry the crystals by gently dabbing with filter paper (or in a warm oven).
Balanced symbol equation:
H₂SO₄ (aq) + CuO (s) → CuSO₄ (aq) + H₂O (l)
Ionic equation:
2H⁺ (aq) + CuO (s) → Cu²⁺ (aq) + H₂O (l)
Making pure samples of soluble salts (using titration)
Soluble salts can also be prepared by reacting an acid with a soluble reactant, typically an alkali like sodium hydroxide or ammonia. This is done using a titration to ensure precise measurements.
- Use a pipette and pipette filler to add a measured volume of alkali to a conical flask.
- Add a few drops of suitable chemical indicator to the alkali.
- Fill the burette with acid and record the starting volume.
- Slowly add acid from the burette to the alkali, swirling the flask to mix.
- Stop adding the acid when the chemical indicator changes colour permanently, indicating the endpoint. Note the final volume reading.
- Repeat until concordant titres (consistent readings) are achieved.
- Complete one more repeat, but this time do not add any chemical indicator. Mix the acid and alkali in the correct proportions and allow the water to evaporate to obtain pure dry crystals of the salt.
Making pure samples of insoluble salts
Insoluble salts are prepared by reacting two soluble salts to form a precipitate. This reaction is called a precipitation reaction.
- Mix solutions of two soluble salts where one contains the desired positive ion and the other contains the desired negative ion.
- Use filtration to separate the precipitate from the solution.
- Wash the precipitate with distilled water to remove any impurities.
- Leave precipitate to dry. If necessary, dry by gently dabbing with filter paper (or in a warm oven).
Worked Example - Making lead iodide
Warning: External video
- Prepare the solutions of two soluble salts
- Prepare an aqueous solution of lead(II) nitrate, Pb(NO₃)₂
- Prepare an aqueous solution of potassium iodide, KI
- Mix the two solutions in a beaker. A yellow precipitate of lead(II) iodide (PbI₂) will form immediately.
- Use filtration to separate the precipitate from the solution.
- Wash the precipitate with distilled water to remove any impurities.
- Leave precipitate to dry. If necessary, dry by gently dabbing with filter paper (or in a warm oven).
Balanced symbol equation:
Pb(NO₃)₂ (aq) + 2KI (aq) → PbI₂ (s) + 2KNO₃ (aq)
Ionic equation:
Pb²⁺ (aq) + 2I⁻ (aq) → PbI₂ (s)
Listen to this page (feature coming soon)
Did you know?
- The process of making salts has been used since ancient times, not only in cooking but also in the preservation of food.
- The term "pH" stands for "potential of hydrogen" and was introduced by Danish chemist Søren Peder Lauritz Sørensen in 1909.
- Lemon juice, a common household item, contains citric acid, which is why it tastes sour and can be used to clean surfaces.
- The first recorded use of litmus, a natural dye derived from lichens, to test for acidity dates back to around 1300 AD.
Why do we care?
- Making salts is fundamental in chemistry for producing a variety of materials used in daily life, including medicines and cleaning products.
- Titration helps in determining concentrations accurately, which is crucial in quality control and environmental monitoring.
- Understanding solubility and precipitation reactions allows for the creation of specific compounds necessary for industrial and laboratory applications.
- Knowledge of acids and bases is crucial for medical treatments, including the production of antacids to relieve indigestion.
Key information
- Acids are substances that release hydrogen ions (H⁺) in water, taste sour, and can corrode metals.
- Bases are substances that neutralise acids to form salt and water, feel slippery, and taste bitter.
- The pH scale measures acidity or alkalinity, ranging from 0 (very acidic) to 14 (very alkaline), with 7 being neutral.
- Indicators, such as litmus paper and universal indicator, are used to determine the pH of a solution.
- Neutralisation reactions occur when acids react with bases to produce salt and water only.
- Concentration refers to the amount of solute dissolved in a given volume of solution, expressed in grams per cubic decimetre (g/dm³) or moles per cubic decimetre (mol/dm³).