
The Periodic Table (GCSE)
KS4 National Curriculum Statement(s) covered
- The modern Periodic Table, showing elements arranged in order of atomic number
- Position of elements in the Periodic Table in relation to their atomic structure and arrangement of outer electrons
- Characteristic properties of metals and non-metals
Skip to:
The Periodic Table is a crucial tool in chemistry, helping scientists to understand and predict the properties of elements. The development of the Periodic Table involved many scientists' contributions over time.
Learn more about specific groups of the table, and general periodicity within the table:
History of the Periodic Table
In 1817, Johann Döbereiner observed that certain groups of three elements, which he called triads, had similar properties. For example, calcium, strontium, and barium formed a triad. Döbereiner noted that the properties of the middle element in each triad were approximately an average of the other two. This early attempt to classify elements suggested that there might be a systematic way to group them based on their characteristics.
In 1864, John Newlands proposed the Law of Octaves. He arranged elements in order of increasing atomic weight and found that every eighth element had similar properties, likening this to the octaves in music. Although Newlands' idea was innovative, it was not widely accepted because it did not apply to all elements.

A significant breakthrough came in 1869 when Dmitri Mendeleev, a Russian chemist, arranged the 63 known elements in order of increasing atomic weight and reordered elements to fit trends in properties. Mendeleev's Periodic Table was unique because he left gaps for elements that he believed had not yet been discovered. He predicted the properties of these unknown elements, and when they were eventually discovered, their properties closely matched Mendeleev's predictions. This accuracy led to the eventual acceptance of his Periodic Table.
Mendeleev used an element's "atomic weight" to organise his table. At that time, "atomic weight" referred to the average mass of an element's atoms. It is important to note that Mendeleev was not aware of isotopes, which lead to some of the confusion in where to place elements in many scientists early tables. Today, we use the term "relative atomic mass" to account for the presence of isotopes, giving a more precise average mass of atoms based on their natural abundance.

The general formulae R²O and RH⁴, use superscripts to show the number of atoms in molecules rather than the current style of using subscripts. For Group 1 ("Gruppe I") this would mean they could form structures like H²O, and Li²O ... which we know to also be true today (H₂O and Li₂O).
The Modern Periodic Table
Today, the Periodic Table is arranged by atomic number, which is the number of protons in an atom's nucleus, rather than atomic weight. This modern arrangement provides a clearer and more accurate representation of the elements.
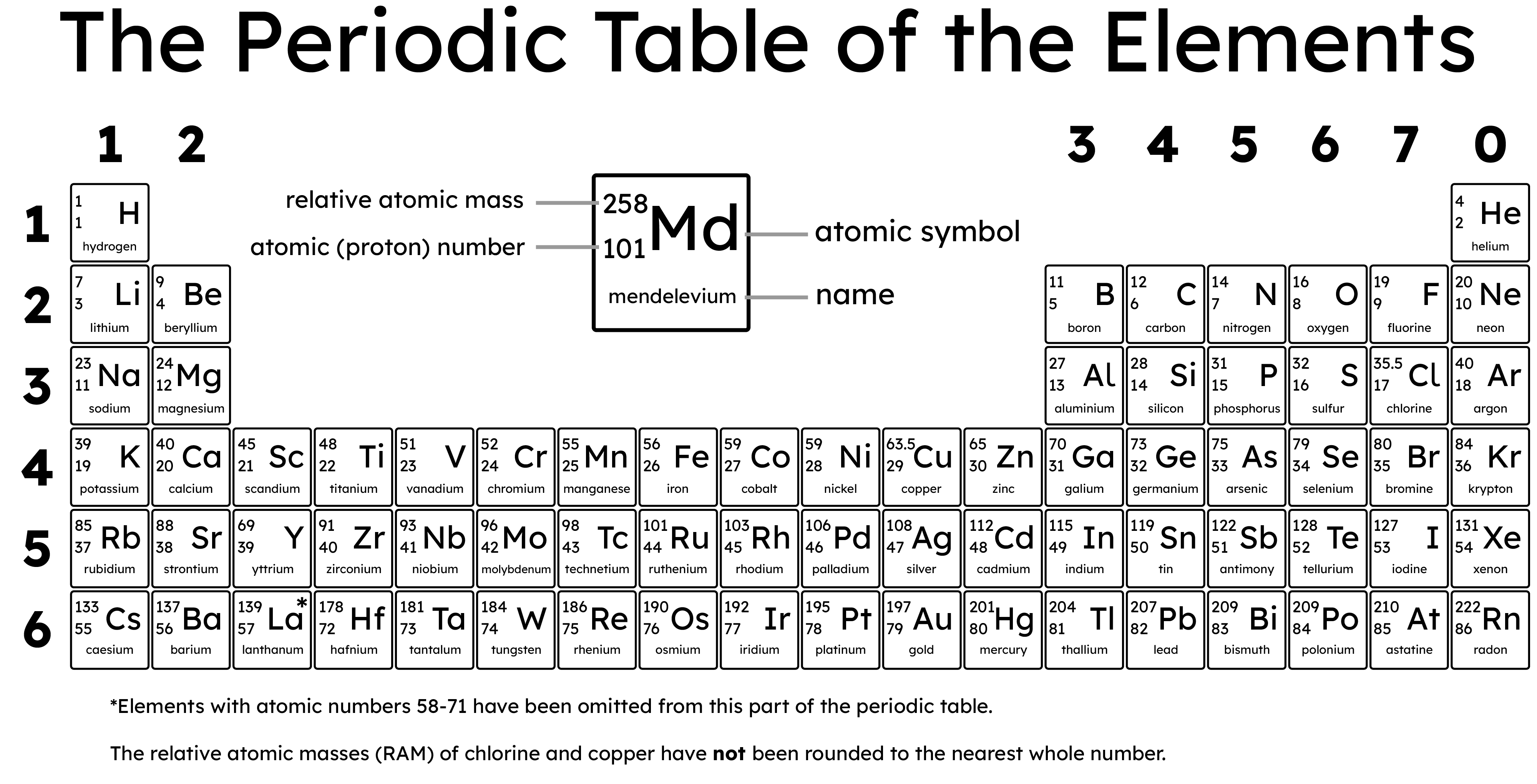
The Periodic Table is divided into periods (horizontal rows) and groups (vertical columns):
- Groups: These vertical columns (numbered 1-7, and 0) contain elements with similar properties and the same number of outer shell electrons. For example, all Group 1 elements have one electron in their outer shell, leading to similar chemical behaviour.
- Periods: These horizontal rows indicate the number of electron shells an element has. For instance, all elements in the second period have two electron shells.
The number of protons in the nucleus of each atom of an element is called the atomic number. The sum of protons and neutrons is the mass number (e.g. if an atom has 6 protons and 6 neutrons, its atomic mass number is 12). The relative atomic mass (often shortened to Ar or RAM) is different to the mass number, and can refer to either:
- The relative atomic mass of a single atom, compared to 1/12th of the mass of a carbon-12 atom
- The weighted average of the masses of all isotopes of an element, based on their natural abundance
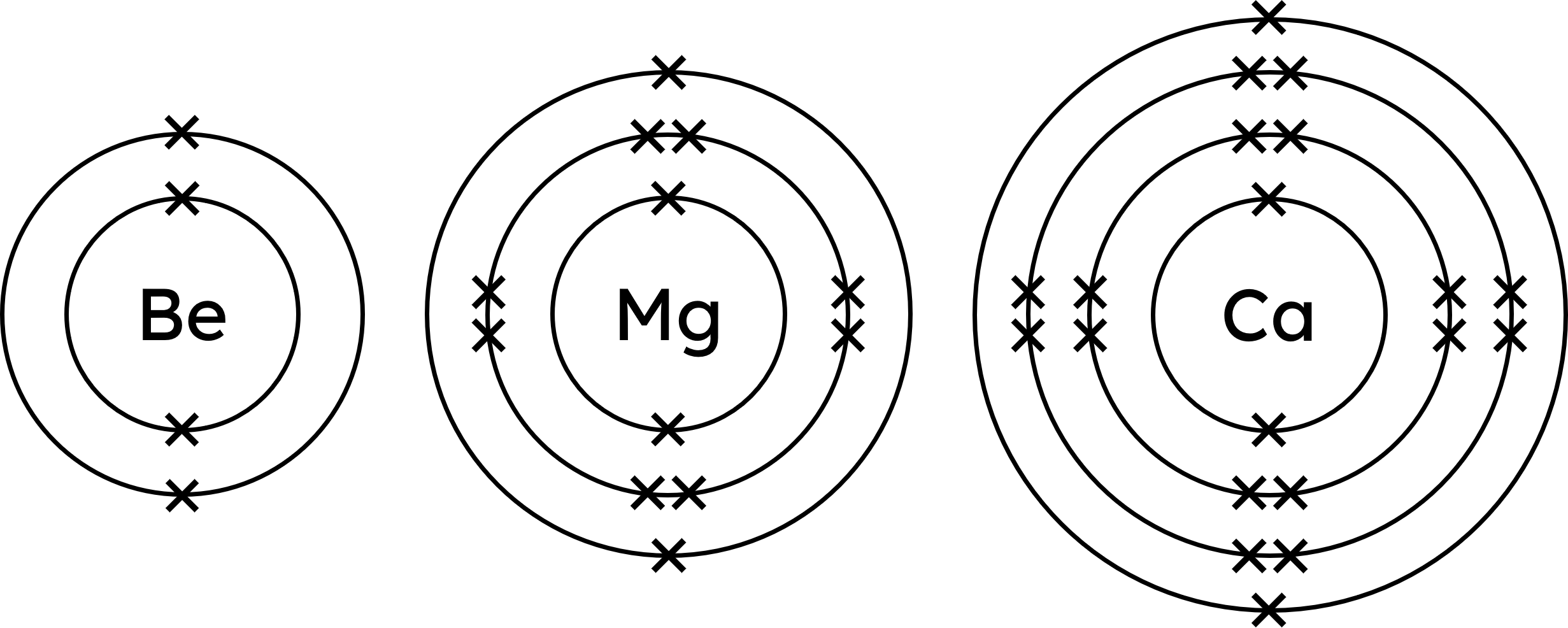
The arrangement of electrons in an atom, known as the electronic configuration, determines its chemical properties. Elements in the same group have the same outer electronic configurations, leading to similar chemical reactions. See the Group 2 elements beryllium, magnesium and calcium below. Each element's atoms have one more shell of electrons than the previous element, but they all have the same number of electrons on their outer shell (two).
Metals and Non-Metals
The Periodic Table is broadly divided into metals and non-metals. This division reflects the differing properties and behaviours of these elements. Metals are typically located on the left and in the centre of the Periodic Table, while non-metals are found on the right side. This arrangement highlights the transition from metallic to non-metallic character as you move across a period (this is known as Periodicity).
Metals, which constitute the majority of the elements, possess a range of distinct physical and chemical properties:
- Have high melting and boiling points.
- Are excellent conductors of heat and electricity
- Are malleable and ductile.

When metals are heated to high temperatures, they melt and can be cast into various shapes. Significant heat is required to transform the metal from the solid to a liquid state, illustrating the strength of the bonds within the metal.
Non-metals, although fewer in number compared to metals, exhibit a different set of properties:
- Have lower melting and boiling points compared to metals.
- Are poor conductors of heat and electricity.
- Are brittle when in the solid state.

Sulfur is a non-metal that exemplifies many of these properties. It has a relatively low melting point compared to metals and is a poor conductor of heat and electricity. Additionally, solid sulfur is brittle and will break rather than bend when subjected to force.
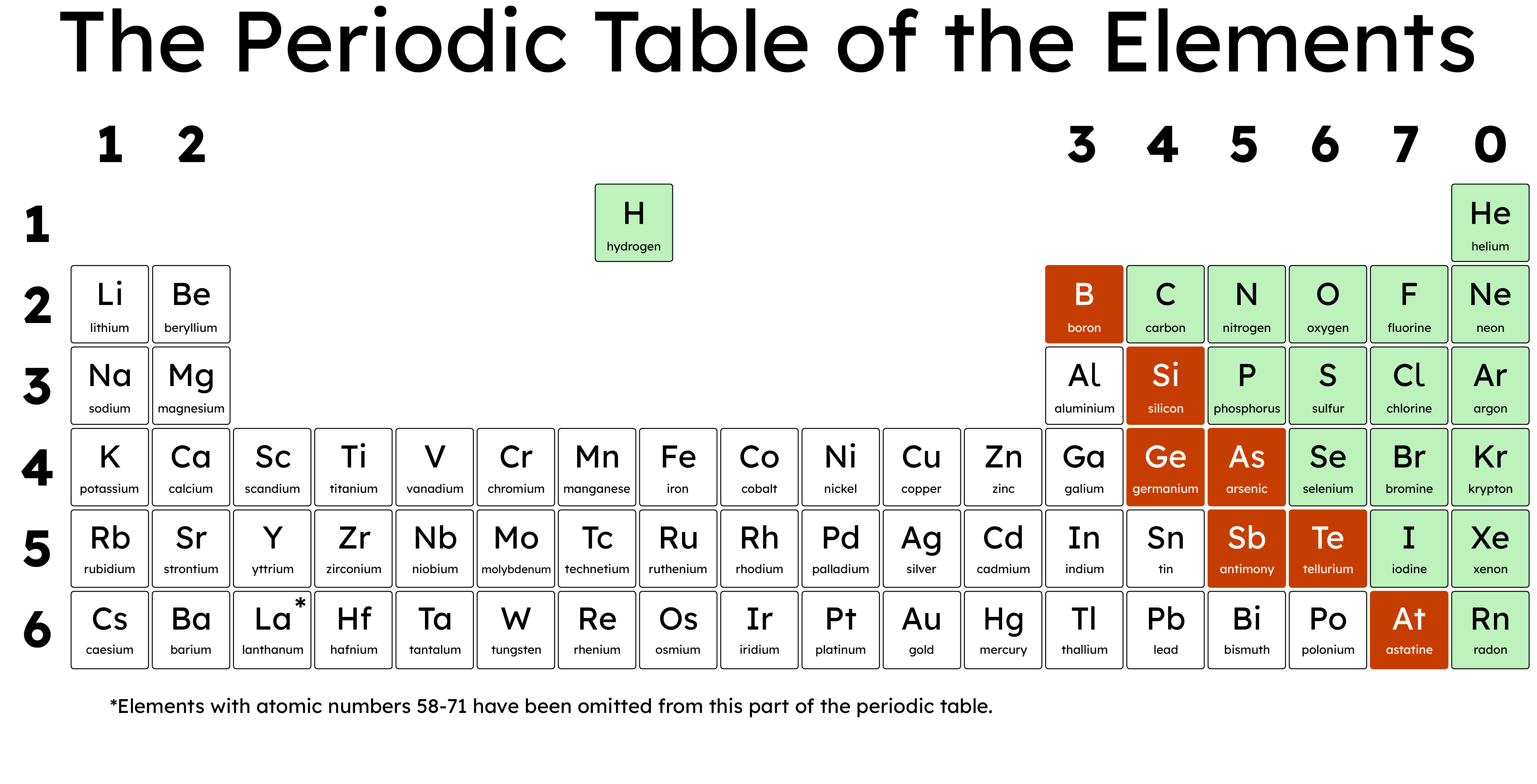
Metalloids exhibit properties that are intermediate between those of metals and non-metals. They are found along a zigzag line on the Periodic Table that separates metals from non-metals. Common metalloids include silicon, germanium, and arsenic. Metalloids can exhibit a mix of metallic and non-metallic properties; they:
- Typically have melting and boiling points that are higher than those of non-metals but lower than those of metals.
- Are semiconductors, meaning they can conduct electricity better than non-metals but not as well as metals.
- Are brittle when in the solid state.

Silicon is a well-known metalloid that is widely used in the electronics industry. It has a moderate melting point and is a good semiconductor, making it essential for the manufacture of electronic devices such as computers and smartphones. Silicon also has a shiny appearance but is brittle and will shatter rather than bend.
Listen to this page (feature coming soon)
Did you know?
- Mendeleev's predictions included elements like gallium and germanium, which fit perfectly into the gaps he left.
- The modern Periodic Table contains 118 confirmed elements, but scientists continue to search for new elements.
- Many heavier elements have been discovered by synthesising them in laboratories. For example, element 118, oganesson, was discovered in 2002 by a team of Russian and American scientists.
- Heavier elements, such as uranium and radium, are radioactive and can decay over time into lighter elements, emitting radiation.
Why do we care?
- The history of thePeriodic Table, from Mendeleev’s early version to the modern table, demonstrates how scientific ideas evolve, showing the importance of critical thinking and innovation.
- Understanding the modernPeriodic Table helps predict element properties and reactions, which is crucial for developing new materials and medicines.
- Knowing the difference between metals and non-metals explains why copper is used in electrical wiring for its conductivity, while non-metals like chlorine are used in disinfectants.
Key information
- The Periodic Table was developed over time, with significant contributions from scientists like Döbereiner, Newlands, and Mendeleev.
- It is now arranged by atomic number, reflecting the number of protons in an atom's nucleus.
- Groups and periods help organise elements with similar properties and electron configurations.
- The Periodic Table distinguishes between metals and non-metals, with metals typically being conductive and malleable, and non-metals being poor conductors and brittle.