
Pure substances and mixtures (GCSE)
KS4 National Curriculum Statement(s) covered
- Distinguishing between pure and impure substances
Skip to:
Understanding the distinction between pure and impure substances is crucial in chemistry. A pure substance is a material that consists of a single element or compound, which means it is not mixed with any other substances. In contrast, a mixture is composed of two or more elements or compounds that are physically combined but not chemically bonded.
Understanding these concepts is essential for mastering separation techniques. Check out the ways we can separate mixtures by visiting the links below:
Pure substances
A pure substance is made up of only one type of element or compound. For example, pure water contains only H₂O molecules. In contrast, an impure substance contains two or more different elements or compounds mixed together.
Pure substances have a uniform composition, meaning they are the same throughout. They have sharp and specific melting and boiling points. For example, pure water boils at 100°C, and freezes at exactly 0°C, at standard atmospheric pressure.
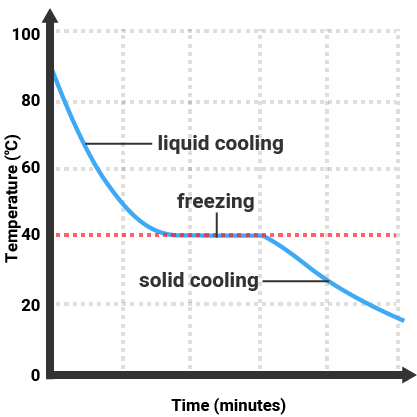
Determining purity can be done by measuring the melting or boiling point of a substance and comparing it to the known values. A pure substance will have a melting or boiling point that matches these known values precisely, while an impure substance will have a range of melting or boiling temperatures.
Mixtures
A mixture consists of two or more elements or compounds that are physically combined but not chemically bonded. This means that the components of a mixture retain their individual properties and can often be separated by physical processes (e.g. filtration, distillation etc.). Examples of mixtures include:
- air (a mixture of nitrogen, oxygen, carbon dioxide, and other gases) - the exact composition can vary slightly depending on location and altitude
- saltwater (a mixture of salt and water)
- alloys like brass (a mixture of copper and zinc)
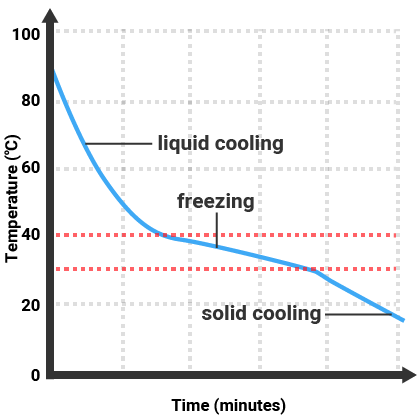
Mixtures do not have sharp melting or boiling points. Instead, they melt or boil over a range of temperatures. For instance, saltwater will start to boil at a temperature slightly above 100°C due to the presence of dissolved salt, and it will continue boiling over a range of temperatures as the concentration of salt increases.
An impure substance is essentially a mixture that contains a predominant substance along with small amounts of one or more other substances. These impurities affect the properties of the predominant substance, such as its melting and boiling points. For example, if salt is dissolved in water, the resulting saltwater is an impure form of water because it contains the impurity of salt.
Formulations
A formulation is a complex mixture where each component is present in a precise amount to achieve specific characteristics and functions. Unlike simple mixtures, formulations are created with a purpose in mind, ensuring that the final product performs as intended.

Formulations are carefully made by mixing different substances in specific proportions to create a product with desirable properties. These mixtures are important in various industries, from pharmaceuticals to cosmetics and household products. Formulations are developed through careful research and testing. Scientists and engineers work together to determine the best combination of ingredients to achieve the desired properties.
Examples of formulations:
- Fuels: Mixtures of hydrocarbons designed for specific energy outputs.
- Cleaning Agents: Combinations of detergents and disinfectants for effective cleaning.
- Paints: Blends of pigments, binders, and solvents to ensure colour, consistency, and durability.
- Medicines: Pharmaceutical formulations containing active ingredients and excipients for safe and effective delivery.
- Fertilisers: Fertiliser formulations contain nutrients like nitrogen, phosphorus, and potassium, essential for plant growth.
- Foods: Formulated for taste, texture, nutrition, and shelf-life. Ingredients are carefully balanced to meet health standards and consumer preferences.
- Alloys: These are mixtures of metals designed to improve properties such as strength, ductility, and resistance to corrosion.
Solutions and suspensions
A solution is a homogeneous mixture, meaning that it has the same composition throughout. Solutions are formed when a solute (the substance being dissolved) is mixed with a solvent (the substance doing the dissolving). When the solvent is water, it is called an aqueous solution (aq).
Solutions do not scatter light, making them clear (transparent). Some solutions might have a colour, or they might be colourless. Examples of solutions include:
- When table salt (sodium chloride) dissolves in water, it breaks into sodium and chloride ions, creating a clear and uniform mixture.
- Sugar crystals dissolve in hot tea to form a sweet solution, with the sugar molecules evenly distributed throughout the liquid.
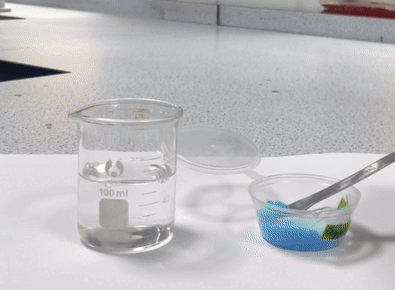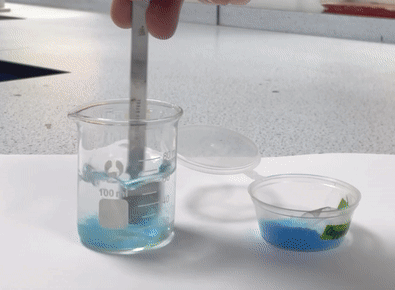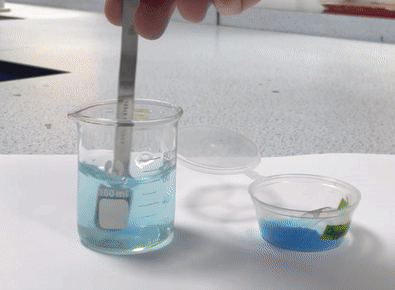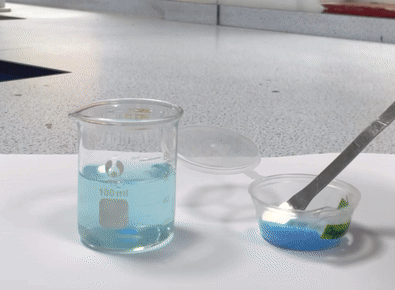
A suspension is a heterogeneous mixture, meaning it has a non-uniform composition. In suspensions, the particles are larger and do not dissolve in the solvent. Instead, they are dispersed throughout the solvent but can settle out over time. Examples of suspensions include:
- In muddy water, soil or clay particles are dispersed in water, creating a mixture that appears cloudy. Over time, the soil particles will settle at the bottom.
- When flour is mixed with water, it forms a suspension. The flour particles are visible and will settle if the mixture is left standing.

Sand mixed with water forms a suspension. The particles in a suspension can settle out over time if left undisturbed and can be separated by filtration. Suspensions scatter light, making them appear cloudy or opaque.
| solutions | suspensions | |
|---|---|---|
| composition | homogeneous | heterogeneous |
| appearance | clear (transparent) | opaque or cloudy |
| stability | solute does not settle | particles settle over time |
| particle size | less than 1 nanometre | over 1 micrometre |
| light scattering | does not scatter light | scatters light |
| separation | cannot be separated by filtration | can be separated by filtration |
Solubility
Solubility is a property of a substance (the solute) to dissolve in a solvent to form a homogeneous mixture called a solution. The maximum amount of solute that can dissolve in a given quantity of solvent at a specific temperature and pressure is known as the solubility of the solute. Solubility is an important concept in chemistry as it influences many reactions and processes, from industrial applications to biological systems.
An unsaturated solution is one where more solute can still dissolve. For example, if you stir some salt into water and all the salt disappears, the solution is unsaturated. This means it can still dissolve more salt if you add it.
A saturated solution is different. This is when the solution contains the maximum amount of solute that can dissolve at a particular temperature and pressure. If you keep adding salt to the water and it starts to settle at the bottom without dissolving, the solution is saturated. It has reached its limit for how much salt it can dissolve.
A supersaturated solution goes even further. This is when a solution contains more solute than it would normally be able to dissolve at a certain temperature. For example, if you heat water and dissolve a lot of salt in it, and then let it cool slowly, the salt might stay dissolved even though the water should not be able to hold that much salt at the lower temperature. This makes the solution supersaturated. However, this type of solution is unstable. If you disturb it, such as by adding a tiny bit more salt or shaking the container, the excess solute can suddenly come out of the solution and form crystals.
Listen to this page (feature coming soon)
Did you know?
- The process of dissolving a solute in a solvent to form a solution is called solvation. In the case of water, it is called hydration.
- The concept of purity dates back to ancient times. Gold coins from ancient civilisations often contained other metals to make them harder and more durable. Modern techniques can determine the purity of these ancient artefacts.
- Diamonds, like gold, have varying degrees of purity, which can significantly influence their value and quality.
- The purity of a substance can be crucial in various industries. For example, in the pharmaceutical industry, the purity of a drug can affect its effectiveness and safety.
Why do we care?
- Identifying pure substances helps ensure the safety and effectiveness of medicines, ensuring they are free from harmful impurities.
- Understanding mixtures and formulations is key in everyday products like toothpaste, which combines ingredients for cleaning, flavour, and fluoride protection.
- Knowledge of solutions and suspensions explains why salt dissolves in water to season food, but oil and water form a suspension, helping in cooking and food preparation.
- Learning about solubility is crucial for creating solutions for tasks like dissolving laundry detergent in water for cleaning clothes.
Key information
- A pure substance contains only one type of element or compound.
- Pure substances have specific melting and boiling points.
- A mixture is a physical combination of two or more elements or compounds.
- Mixtures do not have sharp melting or boiling points; they melt or boil over a range of temperatures.
- An impure substance is a mixture where a predominant substance has impurities, affecting its properties.
- A solution is a homogeneous mixture where the solute is completely dissolved in the solvent.
- A suspension is a heterogeneous mixture where particles are visible and can settle over time.