
Separation techniques (GCSE)
KS4 National Curriculum Statement(s) covered
- Separation techniques for mixtures of substances: filtration, crystallisation, chromatography, simple and fractional distillation
Skip to:
Separation techniques are methods used to separate the components of mixtures, exploiting the physical properties of each component. These techniques are essential for obtaining pure substances and analysing mixtures in various scientific and industrial applications.
Filtration
Filtration is used to separate an insoluble solid from a liquid.
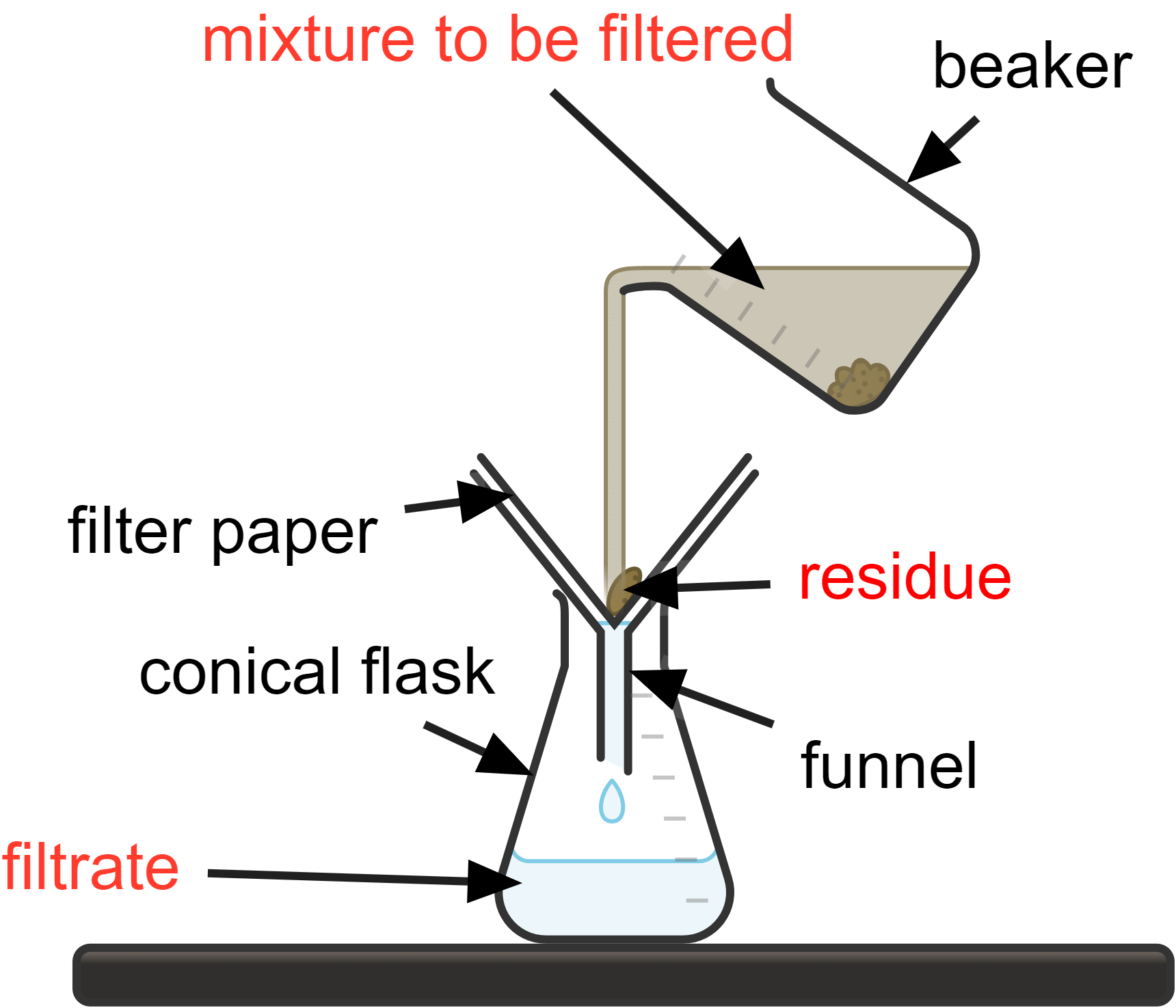
How it works:
- a mixture of liquid and solid is poured through filter paper that is placed in a funnel
- the liquid component, known as the filtrate, passes through the fine pores of the filter paper and collects in a flask below
- the solid component, called the residue, is unable to pass through the filter paper and remains on top of it

A classic example of filtration is separating sand from water. When a mixture of sand and water is poured through filter paper, the sand particles are left behind on the filter paper (residue) while the water drips through (filtrate), resulting in complete separation.
Filtration is widely used in laboratories and industries, such as in water purification systems to remove particulate impurities.
Crystallisation
Crystallisation is used to separate a soluble solid from a solution.
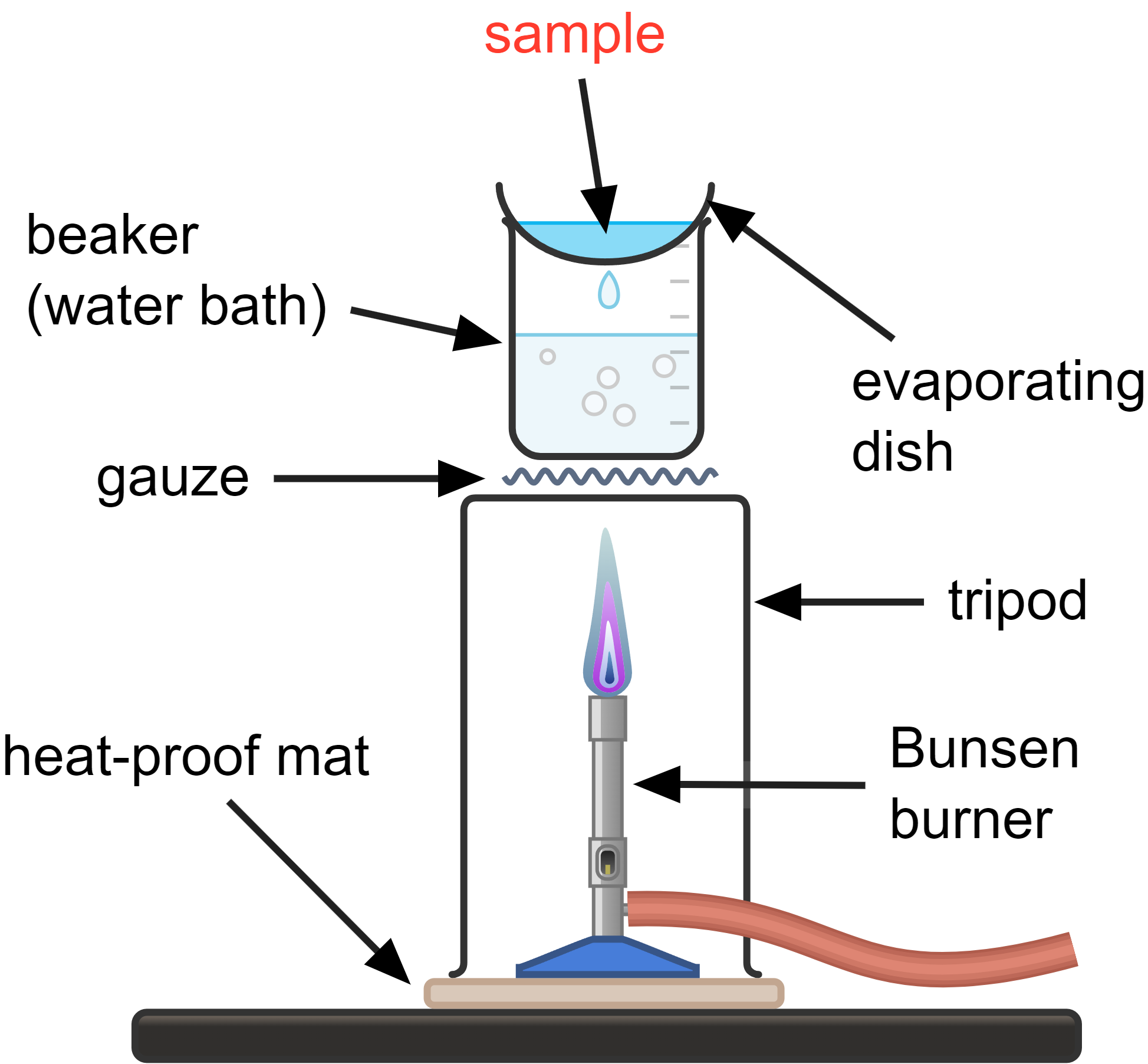
How it works:
- heat the solution (the sample) gently until the solvent begins to evaporate (not boil)
- as the solvent evaporates, the concentration of the solute increases until it starts to form solid crystals (aim to remove no more than half the solvent)
- the remaining solution is left to cool, and then crystallise further
- the crystals may need to be dabbed dry with a paper towel, or left to dry in a warm oven

It is important not to evaporate all the solvent, but only to partially evaporate it. This partial evaporation creates a saturated solution, promoting the formation of large, pure crystals as the solution cools. If all the solvent is evaporated, the solute may form a powder or smaller, less pure crystals. Additionally, completely evaporating the solvent can cause the hot solute to spit or splatter, posing a safety risk.
Salt can be separated from saltwater using crystallisation. By gently heating the saltwater solution, water gradually evaporates, and salt crystals begin to form and grow. These crystals can be collected and dried.
While simple distillation can effectively separate saltwater by evaporating the water and leaving the salt behind, crystallisation is often more practical when the goal is to obtain the salt itself. This is because crystallisation directly yields solid salt crystals, which can be easily collected and used. Distillation would require an additional step to collect the evaporated water and then deal with the salt left behind. Furthermore, evaporating all the solvent can cause the salt to spit or splatter, posing a safety risk. Therefore, when the desired product is the solid solute, crystallisation is the preferred method.
Simple Distillation
Simple distillation separates the solvent from a solution, leaving the solute behind. This works because different substances have different boiling points. The substance with the lower boiling point will vaporise first.
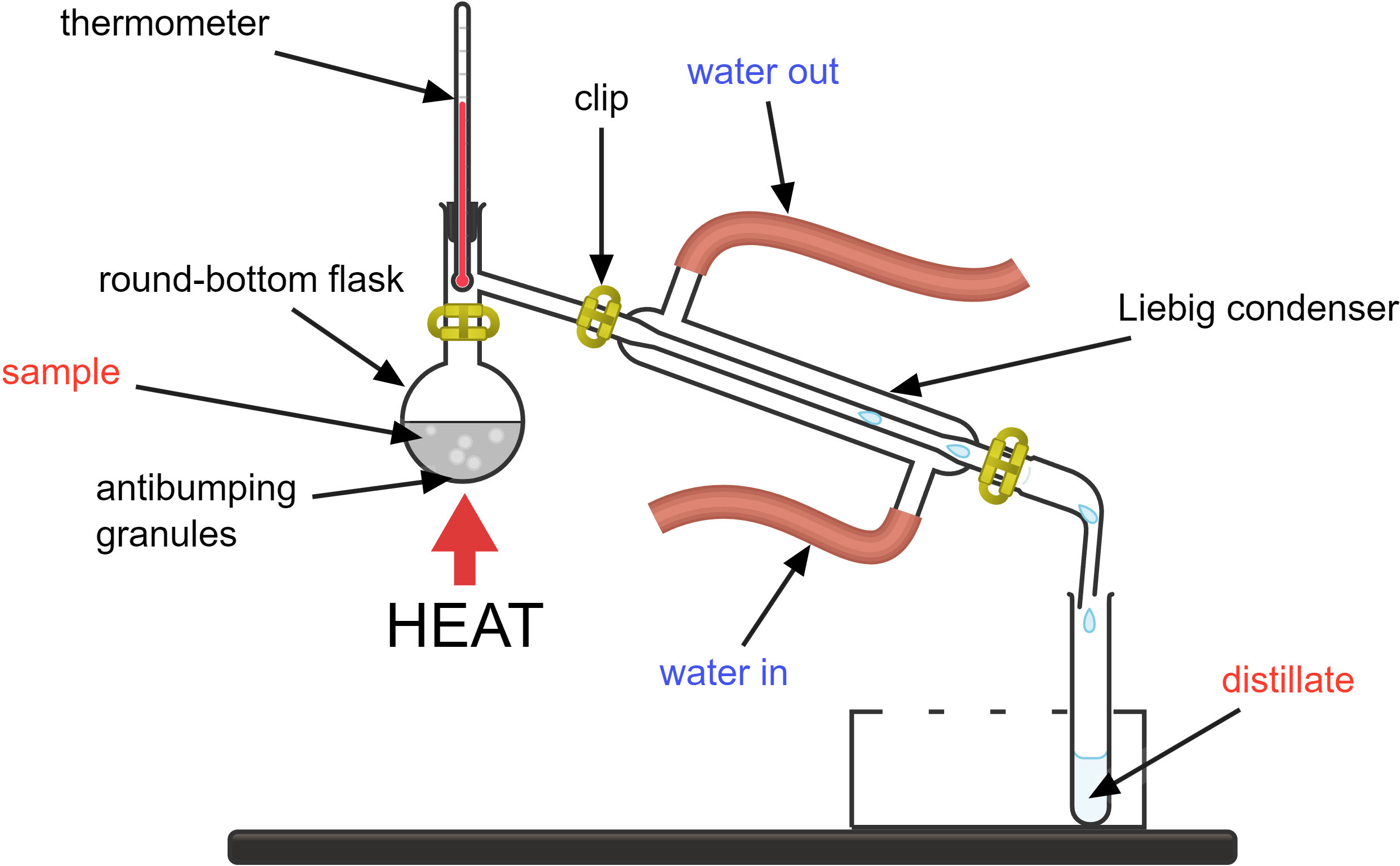
How it works:
- the mixture you want to separate is placed in the round-bottom flask
- the round-bottom flask is heated; as the mixture heats up, the component with the lower boiling point will start to vaporise first
- the thermometer monitors the temperature of the vapours; this helps ensure you know which component is boiling if you are separating a mixture with multiple components
- the vapour travels through the condenser, which is a long tube surrounded by a cooling jacket
- cold water enters the condenser jacket through the “water in” tube and exits through the “water out” tube
- the cold water absorbs heat from the vapour, causing it to condense back into a liquid
- the condensed liquid (distillate) drips out of the condenser and is collected in a receiving flask or beaker
- the substance left behind in the round-bottom flask is the component(s) with higher boiling points
Antibumping granules are used in distillation to ensure smooth boiling by providing nucleation sites, which helps prevent the formation of large bubbles and sudden boiling (bumping) that can cause splashing and uneven heating.

In typical laboratory setups for distillation, the systems are not fully enclosed to avoid pressure build-up, which can be dangerous. Instead, they are designed to allow vapours to escape through a condenser and condense back into liquid form.
Water can be separated from saltwater using simple distillation. By heating the saltwater, water evaporates and is then condensed into a separate container (the distillate), leaving the salt behind. This process is used in desalination plants to produce potable water from seawater.
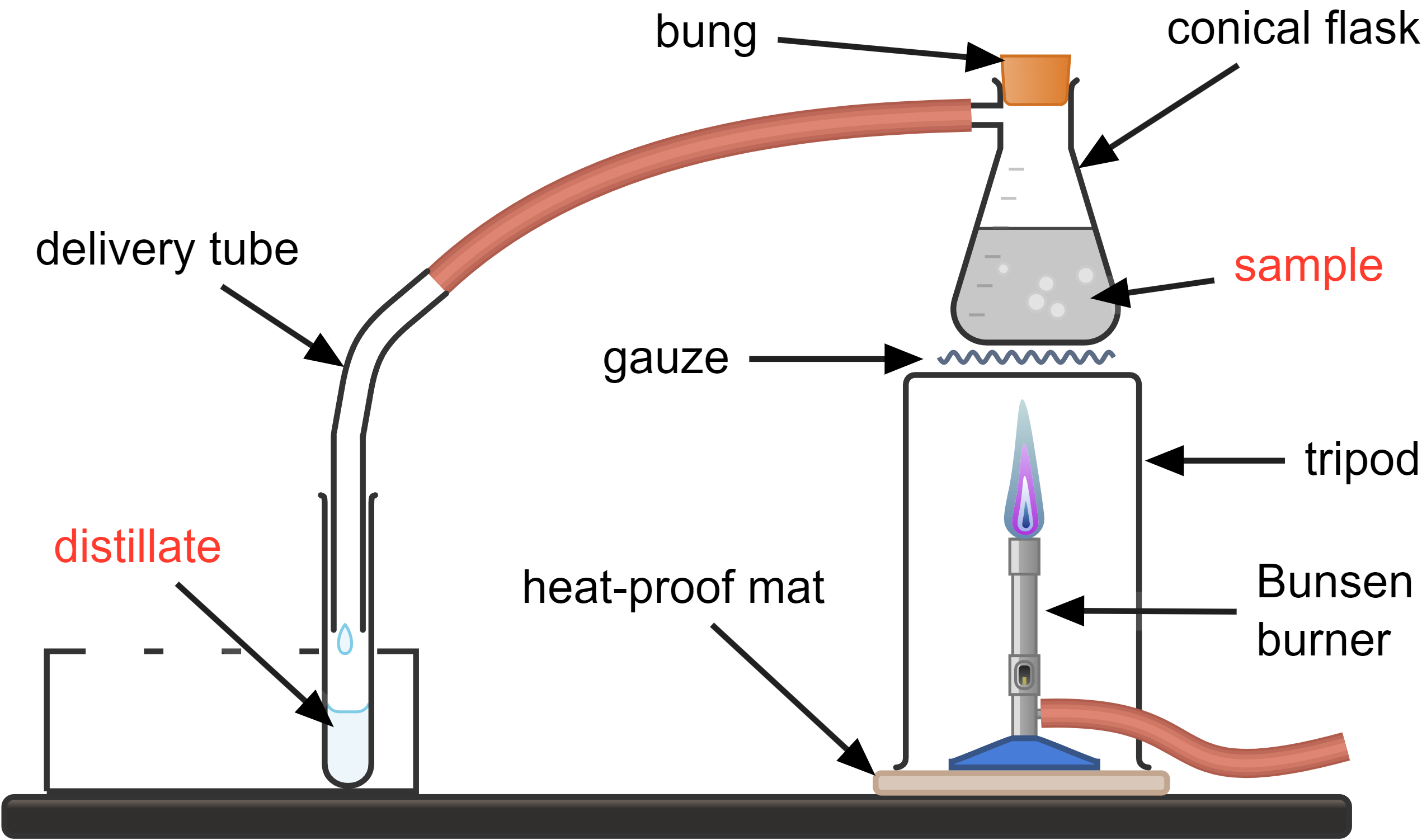
The image shows a basic setup for simple distillation, which includes a Bunsen burner, a conical flask containing an ink-water mixture, a glass delivery tube, and a test tube to collect the distillate. This arrangement, while functional, is less efficient compared to a setup using a Liebig condenser.
In this basic simple distillation process, the ink-water mixture is heated by the Bunsen burner, causing the water (with a lower boiling point) to vaporise. The vapour then travels through the glass tube and cools as it moves through the air, eventually condensing back into liquid form in the test tube. This method relies on ambient air for cooling, which is less efficient and may lead to incomplete condensation and loss of vapour.

Fractional Distillation
Fractional distillation is used to separate multiple liquids from each other. Fractional distillation operates on the same principle as simple distillation but is used to separate mixtures of liquids with closer boiling points. It involves the use of a fractionating column, which provides a large surface area for repeated condensation and evaporation cycles.
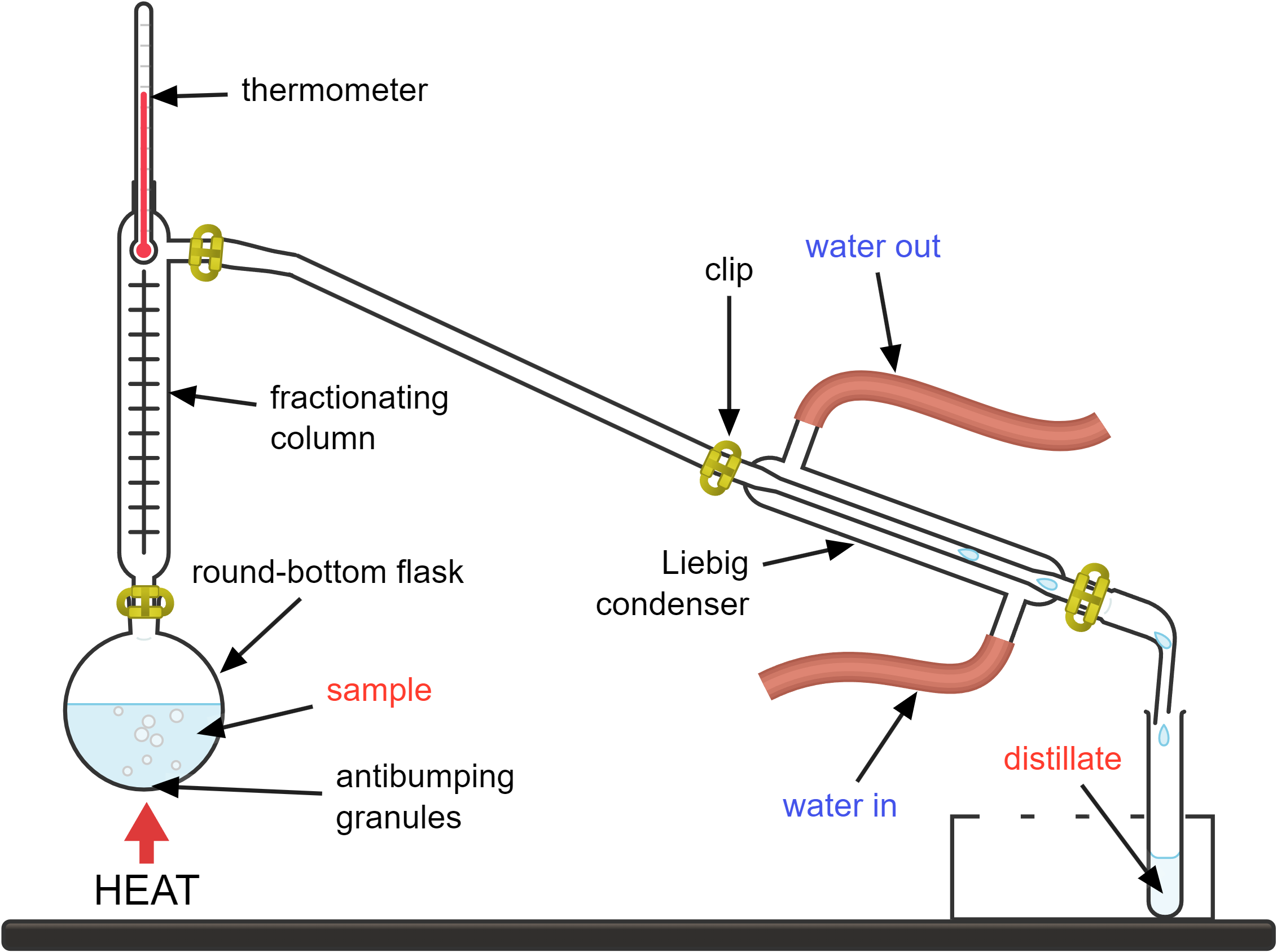
How it works:
- the mixture is heated, and as the temperature increases, the components with lower boiling points start to evaporate
- the vapour rises up the fractionating column, which is filled with materials like glass beads or plates providing a large surface area for condensation and evaporation
- as the vapour rises, it cools and condenses on the surfaces within the column
- the condensed liquid then evaporates again as it is heated by the rising vapour from below; this cycle of condensation and evaporation happens multiple times as the vapour moves up the column
- at the top of the column, the vapour with the lowest boiling point component reaches the condenser, whereas the vapour with the highest boiling point condenses at the bottom.
Each time the vapour condenses and evaporates, it becomes richer in the component(s) with the lower boiling point(s). This is because the higher boiling point components tend to condense and fall back into the flask, while the lower boiling point components continue to rise
For complex mixtures, this process does not result in pure individual components but in simpler mixtures of substances with similar boiling points, known as fractions. These fractions can then be further processed if needed to obtain purer components.
| aspect | basic simple setup | liebig condenser setup | fractional distillation |
|---|---|---|---|
| cooling efficiency | relies on ambient air; less efficient | uses water circulation; highly efficient | uses water circulation; highly efficient |
| temperature control | lacks precise control | precise control | precise control |
| speed and yield | slower process, lower yield | faster process, higher yield | slower process, highest purity and yield |
| safety | open flame, risk of fire; and hot vapour escape | safer with electric heating mantle and controlled vapour flow | safer with electric heating mantle and controlled vapour flow |
| separation efficiency | suitable for simple mixtures | suitable for simple mixtures | suitable for complex mixtures |
Chromatography
Chromatography is used to separate mixtures of soluble substances like inks or dyes. It is comprised of two stages: the stationary phase, and the mobile phase.
Unlike the other separation techniques that separate mixtures into their individual components, chromatography is often used as an analytical tool rather than a separation method. It helps identify whether a substance is pure or a mixture and can show the presence of various components within a sample.
This makes chromatography invaluable in fields like forensic science, biochemistry, and environmental analysis, where identifying and quantifying the components of a mixture is essential.
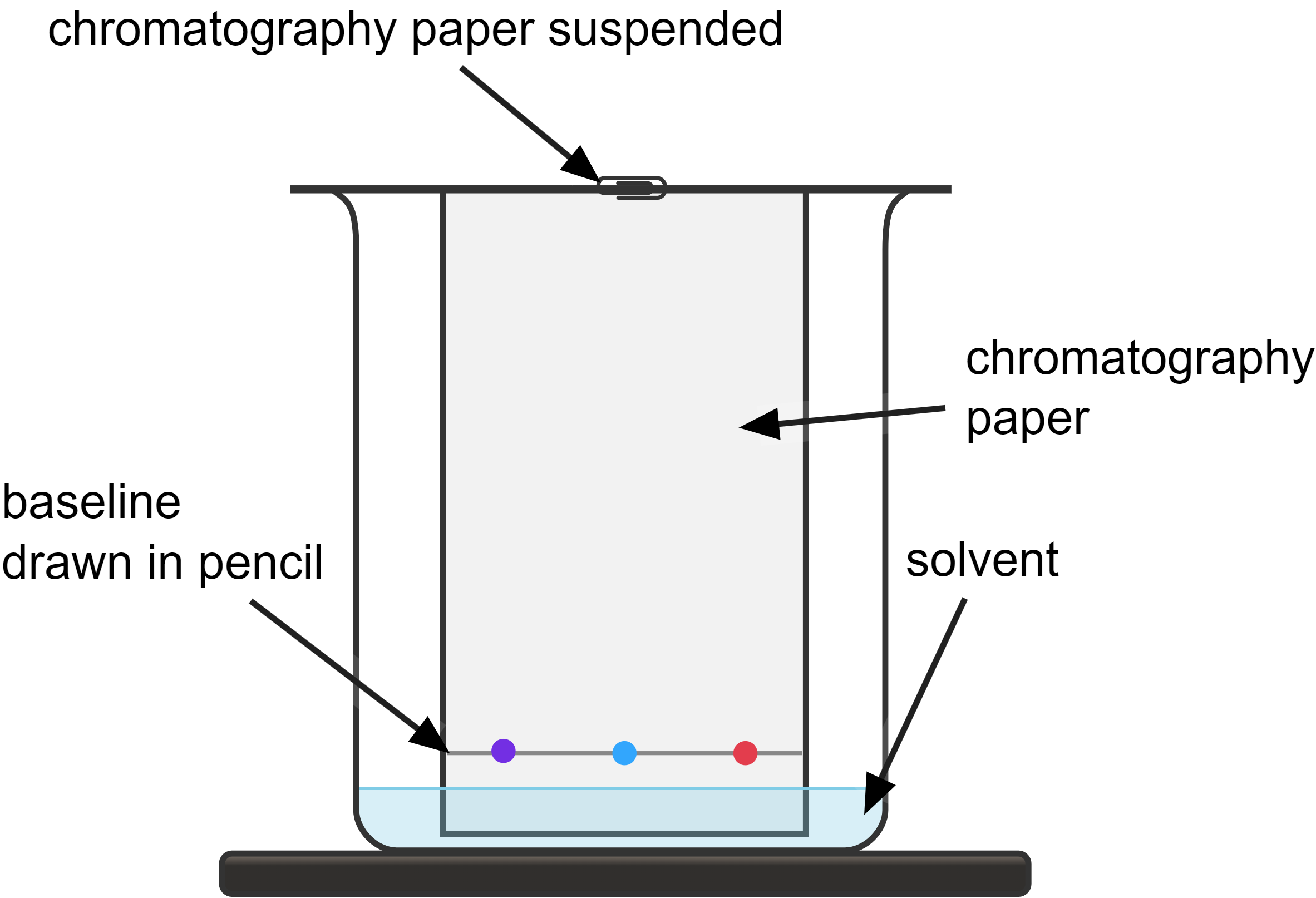
How paper chromatography works:
- involves placing a small sample of the mixture on a stationary phase (such as chromatography paper) and then allowing a solvent (the mobile phase) to move through it
- as the solvent travels up the paper, it 'carries' the different components of the mixture with it (as they dissolve in the solvent)
- different substances in the mixture move at different rates, depending on their solubility in the solvent and their affinity for the stationary phase
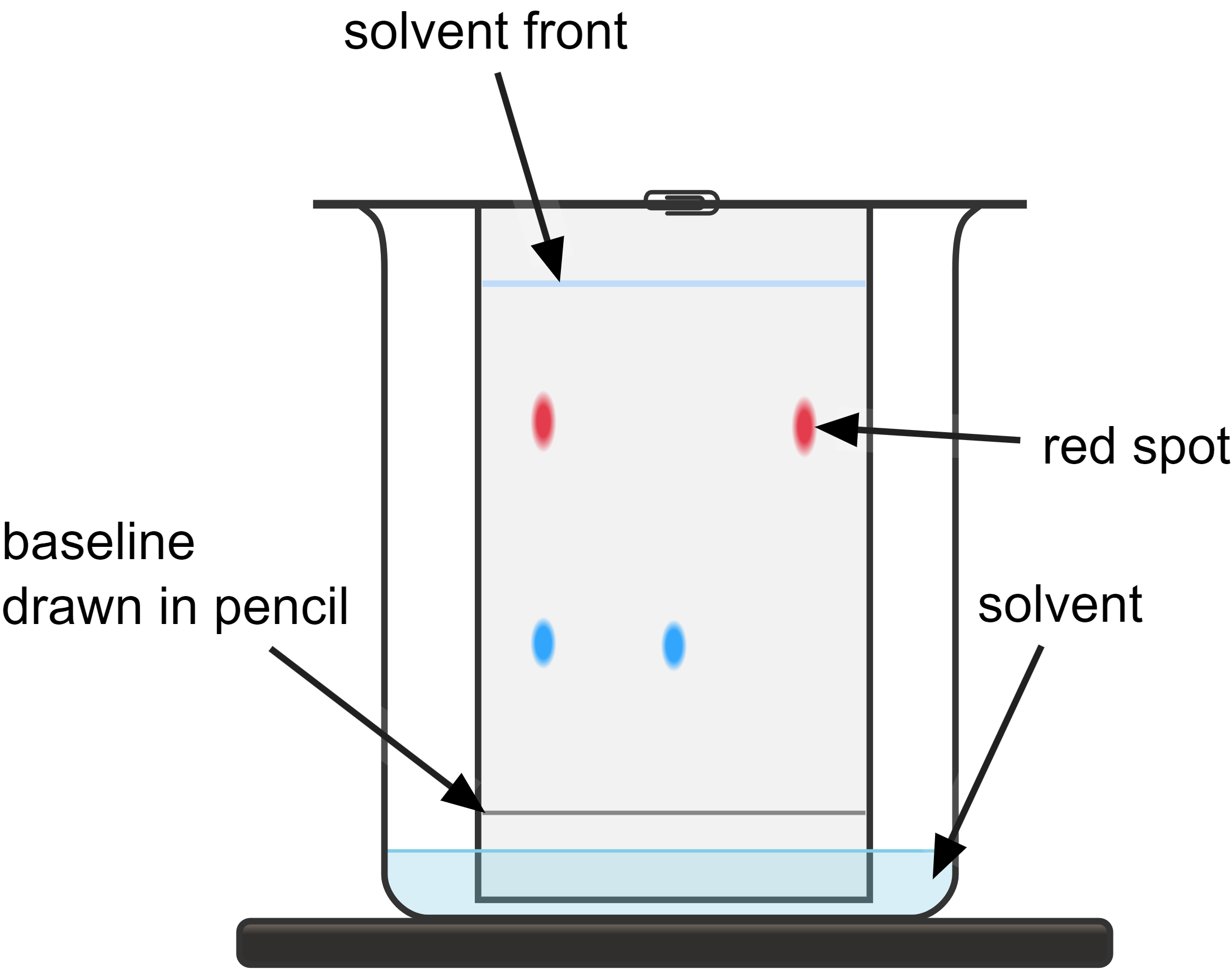
Observe the chromatogram to identify the number of spots and their positions. Pure substances will produce only one spot on the chromatogram. In contrast, impure substances or mixtures will produce multiple spots, as the different components of the mixture separate based on their affinities for the solvent and the paper.
Worked Example - Interpreting a chromatogram
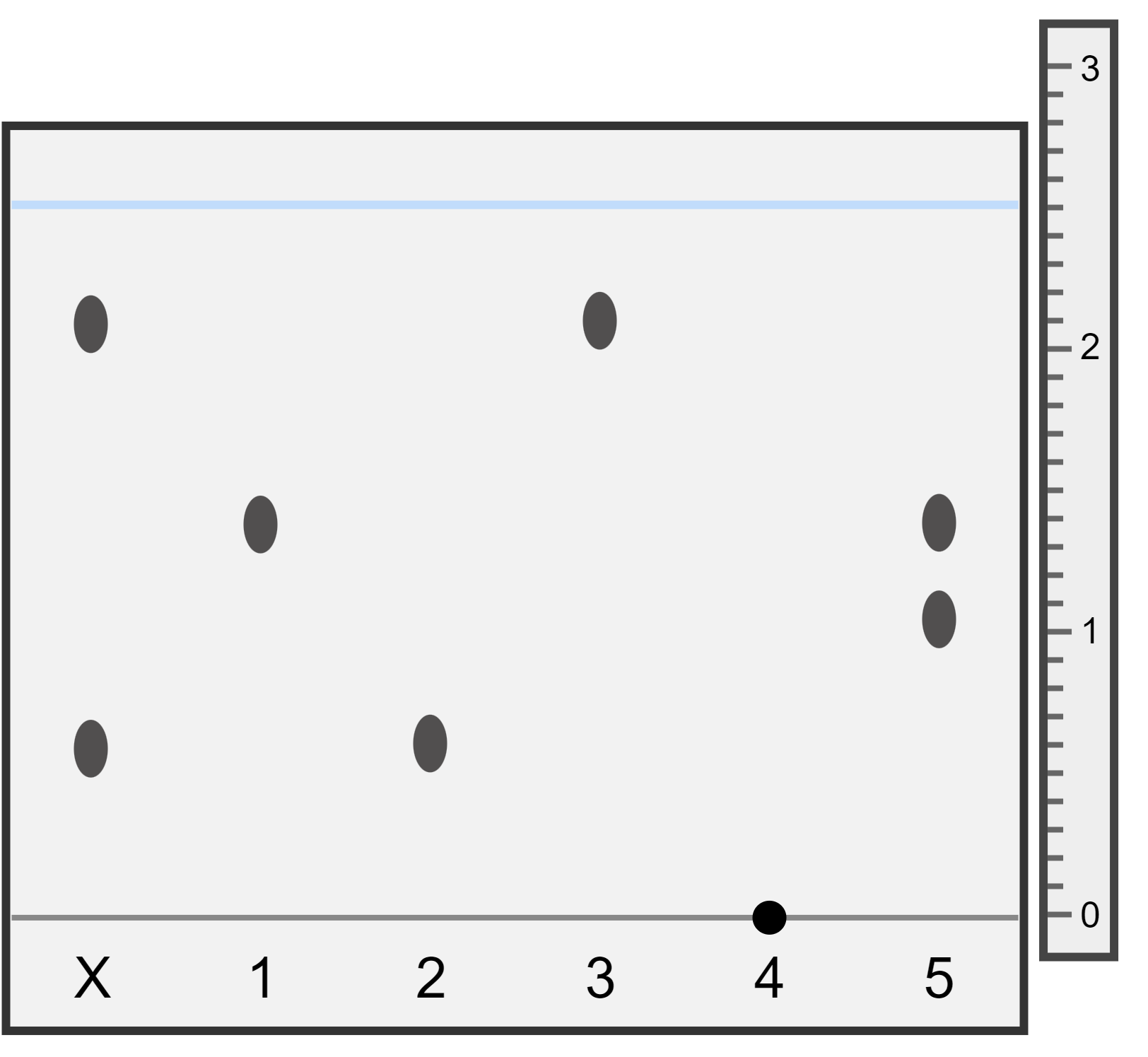
You are provided with the following chromatogram and ruler which shows the distances (in cm) travelled by substances X, 1, 2, 3, 4, and 5 during chromatography. Answer the following questions:
- Which substances are found in sample X?
- Which substances 1-5 are pure?
- What happened to substance 4?
First draw a horizontal line through the centre of each of the spots found in substance X. These lines cross over the spots found in substance 2 and substance 3, so this means substance X is made up of substance 2 and 3.
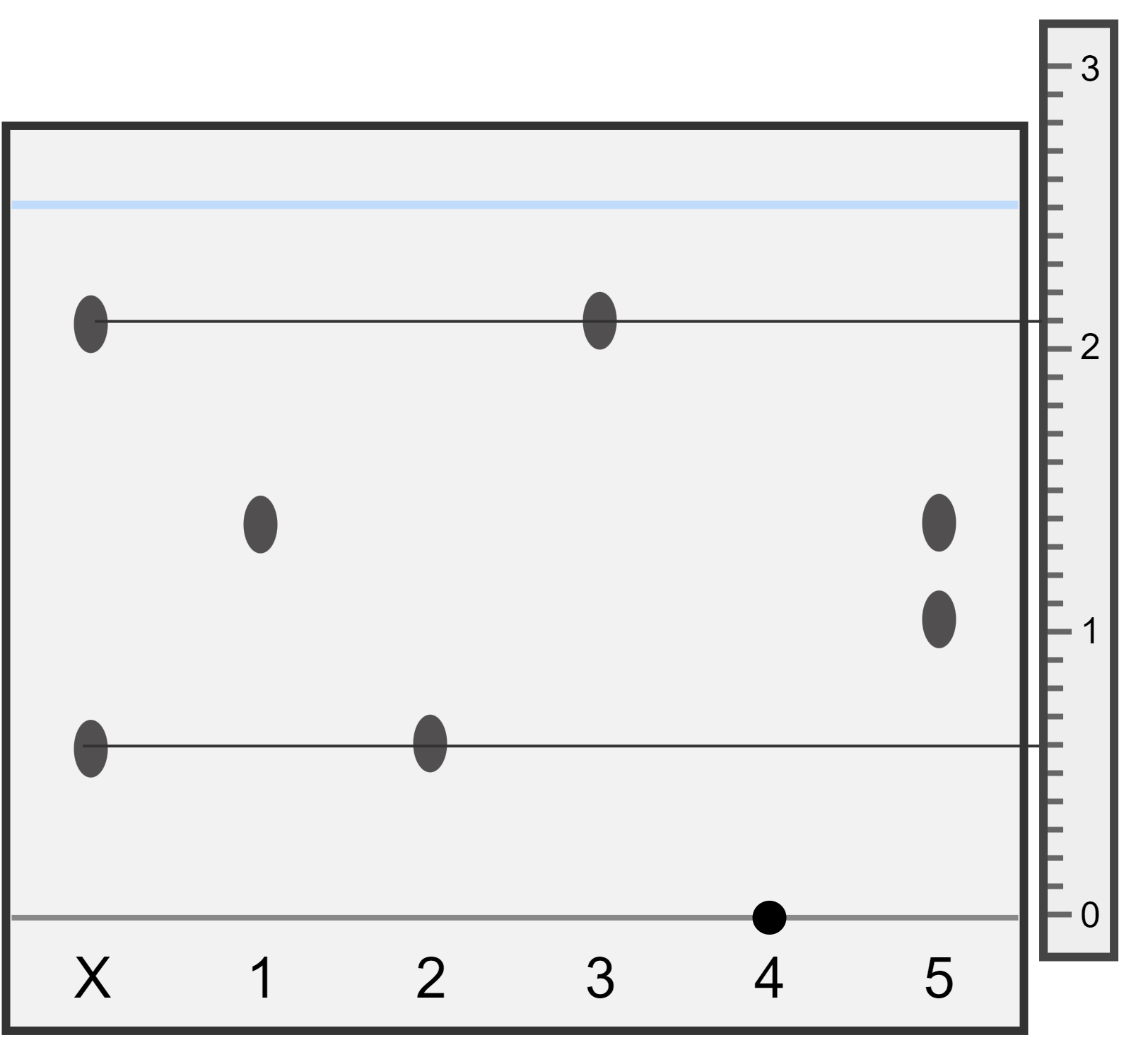
Substances 1, 2, and 3 contain only one spot on the chromatogram - this means they are pure. Substance 5 is made of two spots, so is impure/a mixture.
Substance 4 was insoluble in the solvent used, and didn't move up the paper. We do not know if it is pure or a mixture. A different solvent should be used that substance 4 is soluble in.
Avoiding errors in your paper chromatography analysis:
- make sure to draw the baseline in pencil, otherwise, the ink from a pen would also dissolve in the solvent
- place small spots of the samples to be analysed on the baseline using a capillary tube or a pipette, this is to avoid overlapping during the separation process
- it is crucial that the solvent level is below the baseline at the very beginning to prevent the samples from dissolving directly into the solvent
- you may need to seal the chamber to prevent the solvent from evaporating
- you should not disturb the chamber whilst the chromatography is running, as this will cause the solvent to splash and affect the resulting chromatogram
- remove the chromatogram before the solvent reaches the to and mark the solvent front with a pencil immediately, as it will start to evaporate quickly
Paper chromatography (PC), thin-layer chromatography (TLC), and gas chromatography (GC) are all techniques used to separate and analyse compounds, but they differ significantly in their methods and applications.
| feature | PC | TLC | GC |
|---|---|---|---|
| stationary phase | paper | thin layer of adsorbent (e.g., silica gel) | column packed with solid or liquid-coated solid |
| mobile phase | liquid solvent | liquid solvent | inert gas (e.g., helium, nitrogen) |
| sample application | spotted on paper | spotted on plate | vaporised and injected into column |
| separation mechanism | capillary action (liquid moves up paper) | capillary action (liquid moves up plate) | boiling points and interaction with column |
| common applications | small molecules (amino acids, sugars, pigments) | wide range of compounds (organic compounds, drugs) | volatile and semi-volatile compounds (gases, solvents, essential oils) |
| speed | moderate | fast | very fast |
| resolution | low | high | very high |
| advantages | simple, cheap, minimal equipment needed | higher resolution, faster, wide range of uses | high resolution, sensitive, quick, quantitative analysis |
| disadvantages | lower resolution | requires careful handling and preparation | needs specialised equipment, not for non-volatile or heat-sensitive compounds |
The Rf value, or Retention factor, is a numerical value that represents the relative distance travelled by a substance on a chromatogram. It is a way to quantify the movement of substances during chromatography, making it easier to identify and compare different substances.
Example applications:
- Forensic science: In forensic labs, Rf values can help identify substances in crime scene samples by comparing them to known standards.
- Food industry: Rf values are used to ensure the quality and consistency of food products by identifying and quantifying the presence of specific additives or contaminants.
- Environmental analysis: Environmental scientists use Rf values to detect pollutants in water and soil samples by comparing them to known pollutants.
To calculate the Rf value, you need to measure the distance travelled by the substance and the distance travelled by the solvent front (the leading edge of the solvent) from the baseline (the starting point). The Rf value will always be the same for each substance (when the same solvent is used), and an Rf value will always have a value of less than 1. The formula for calculating the Rf value is:
Here are the steps to calculate the Rf value:
- Place a small spot of the sample mixture on the baseline of the chromatography paper. Allow the solvent to move up the paper by capillary action.
- When the solvent has travelled a sufficient distance, remove the paper from the solvent and immediately mark the solvent front with a pencil.
- Measure the distance from the baseline to the centre of each separated substance spot. Also, measure the distance from the baseline to the solvent front.
- Use the formula above to calculate the Rf value for each substance spot.
Worked Example - Calculating Rf values
Calculate the Rf values of the spots found in substance X on the chromatogram below. Use the ruler where the scale is in centimetres (cm).

The spot that has travelled the least distance (the bottom spot) has travelled 0.6 cm. The distance the solvent front has travelled is 2.5 cm.
The spot that has travelled the furthest distance (the top spot) has travelled 2.1 cm. The distance the solvent front has travelled is 2.5 cm.
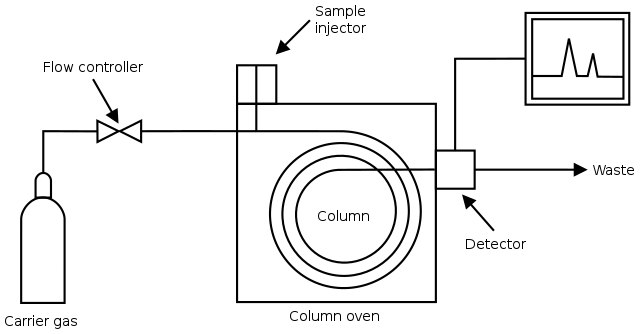
Gas chromatography (GC) works by injecting a sample into a stream of inert carrier gas, which transports the sample through a column coated with a stationary phase. As the sample travels through the column, different components interact with the stationary phase and move at different rates, causing them to separate.

The separated components exit the column and are detected, producing a chromatogram that shows peaks corresponding to each component's retention time, allowing identification and quantification. The oven temperature can be adjusted to help with the separation process by vaporising the compounds at different points.
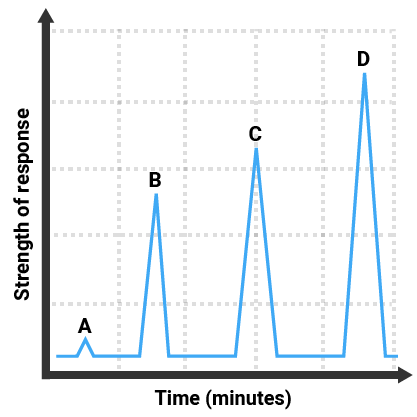
The gas chromatogram here shows that:
- four different substances were in the mixture (there are four peaks)
- substance A was present in the smallest amount (it has the smallest peak)
- substance D was present in the greatest amount (it has the largest peak)
Listen to this page (feature coming soon)
Did you know?
- Ancient Egyptians used a form of distillation to create perfumes and aromatic oils over 5,000 years ago.
- Astronauts use a high-tech version of distillation to recycle water from urine, sweat, and other sources on the International Space Station.
- Crystallisation is not only used in chemistry labs but also in the food industry to produce sugar and salt in their purest forms.
Why do we care?
- Filtration is used to purify drinking water, ensuring it is safe to consume by removing impurities and pathogens.
- Crystallisation helps in the production of pure salt from seawater, used in cooking and food preservation.
- Simple distillation is used to produce alcoholic beverages, and fractional distillation is essential for refining crude oil into fuels like petrol and diesel.
- Chromatography is crucial for detecting drugs in urine samples and ensuring the quality of food and medicines by identifying and separating components.
Key information
- Filtration separates an insoluble solid from a liquid using filter paper.
- Crystallisation separates a soluble solid from a solution by forming crystals as the solvent evaporates.
- Distillation separates substances based on boiling points, with simple distillation used for solvents and fractional distillation for liquids with close boiling points.
- Chromatography separates mixtures of soluble substances by their movement through a stationary phase with a mobile phase.
- The Rf value in chromatography is the ratio of the distance travelled by a substance to the distance travelled by the solvent front.