
Water (GCSE)
KS4 National Curriculum Statement(s) covered
- Distinguishing between pure and impure substances
- The Earth’s water resources and obtaining potable water
Skip to:
Water is essential for life. For humans, drinking water must have sufficiently low levels of dissolved salts and microbes to be considered safe. This type of water is called potable water. Unlike pure water, which contains only H₂O molecules, potable water contains various dissolved substances. The methods used to produce potable water vary depending on the available water supplies and local conditions.
- Pure water: contains only H₂O molecules with no dissolved substances.
- Potable water: contains low levels of dissolved salts and microbes, making it safe to drink but not chemically pure.
Water used for chemical analysis must be very pure to avoid contamination of the results. This type of water is called distilled or deionised water. Both methods ensure that the water is free from minerals, salts, and other contaminants that could interfere with chemical tests.
- Distilled water is made by boiling water and then condensing the steam back into a liquid, which removes most impurities.
- Deionised water is produced by passing water through a special resin that removes ions and other dissolved substances.
If tap water, which contains dissolved substances (salts), is used for chemical analysis instead of pure water, unexpected precipitates may form. These precipitates can hide the correct result of the analysis, making it inaccurate.
The Water Cycle

The water cycle is a natural process that continuously recycles water on Earth. It involves the following stages:
- Evaporation: Water from oceans, lakes, and rivers is heated by the sun and turns into water vapour, rising into the atmosphere.
- Condensation: As the water vapour rises, it cools and turns back into liquid droplets, forming clouds.
- Precipitation: When the clouds become heavy, the water falls back to the Earth as rain, snow, sleet, or hail.
- Collection: The precipitated water collects in bodies of water like rivers, lakes, and oceans, or it infiltrates the ground, replenishing groundwater supplies.
This cycle is crucial for providing fresh water with low levels of dissolved substances, which can be processed into potable water.
Producing Potable Water
In the United Kingdom, rain provides water with low levels of dissolved substances, known as freshwater. This water collects in the ground, lakes, and rivers.

Freshwater from rain often seeps through the soil and collects in aquifers, which are underground layers of rock that hold water. Groundwater from aquifers is a common source of drinking water because it is naturally filtered as it moves through layers of soil and rock. This natural filtration removes many impurities, making groundwater relatively clean.
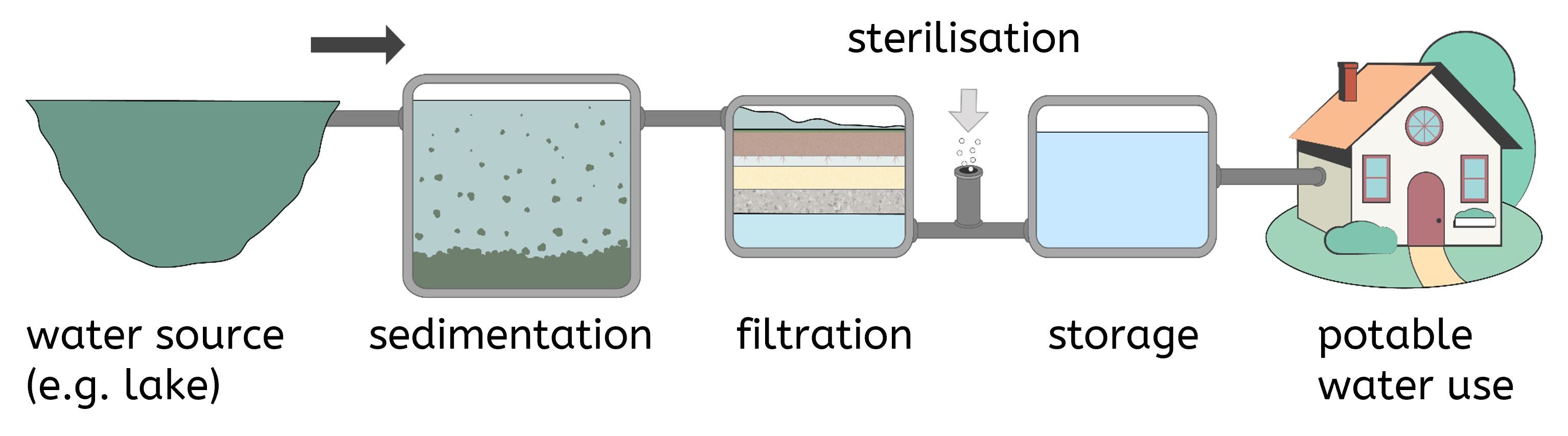
To produce potable water from fresh water sources, the following steps are typically followed:
- Choosing an appropriate source: Selecting a source of fresh water with the lowest levels of contaminants.
- Screening: The first step in water treatment is screening, which involves passing the water through metal grids or meshes to remove large debris such as leaves, twigs, and litter. This prevents blockages and protects the subsequent treatment stages from damage.
- Sedimentation: After screening, the water undergoes sedimentation. In this process, water is stored in large tanks where heavy insoluble particles, such as sand and silt, settle to the bottom due to gravity. This step reduces the amount of solid matter in the water.
- Filtration: The water is then passed through filter beds composed of layers of sand, gravel, and sometimes charcoal. Filtration removes smaller particles that were not eliminated during sedimentation. These particles can include fine sand, silt, and some microorganisms.
- Sterilisation: The final step is sterilisation, where the water is treated to kill any remaining microorganisms. Sterilising agents used for potable water include chlorine, ozone, and ultraviolet (UV) light. Each method has its advantages and disadvantages.
| sterilisation method | advantages | disadvantages |
|---|---|---|
| chlorine | effective against bacteria and viruses, continues to disinfect water from the treatment plant to your tap | can form harmful by-products, less effective against some protozoa |
| ozone | kills bacteria, viruses, and protozoa quickly, leaves no harmful residues | expensive and complex, no continued protection after treatment |
| UV light | kills bacteria and viruses effectively, no harmful by-products | does not protect against new contaminants after treatment |
Chlorine is widely used because it remains active in the water as it travels through pipes from the treatment plant to homes. This means it can kill any new bacteria or viruses that enter the water after it leaves the treatment plant. However, chlorine can react with natural organic matter in the water to form potentially harmful by-products like trihalomethanes (THMs).
UV light kills bacteria and viruses by damaging their DNA, but it only works at the treatment plant. Once the water leaves the plant, UV light does not provide any continued protection against new contaminants that might enter the water as it travels through pipes.
Fluoridation is the addition of fluoride to drinking water to reduce tooth decay. It is a topic of debate due to the potential benefits and risks. Supporters argue that fluoridation reduces cavities and dental health problems, particularly in children. Studies have shown that communities with fluoridated water have lower rates of tooth decay. However, opponents raise concerns about potential health risks, such as dental fluorosis (mottling of teeth) and possible links to other health issues, though scientific evidence on these risks is not conclusive. The debate also includes ethical considerations about whether it is appropriate to medicate a population through the water supply without individual consent. In the United Kingdom, fluoridation of water supplies is not widespread and include parts of the West Midlands, North East of England (and a few other regions).

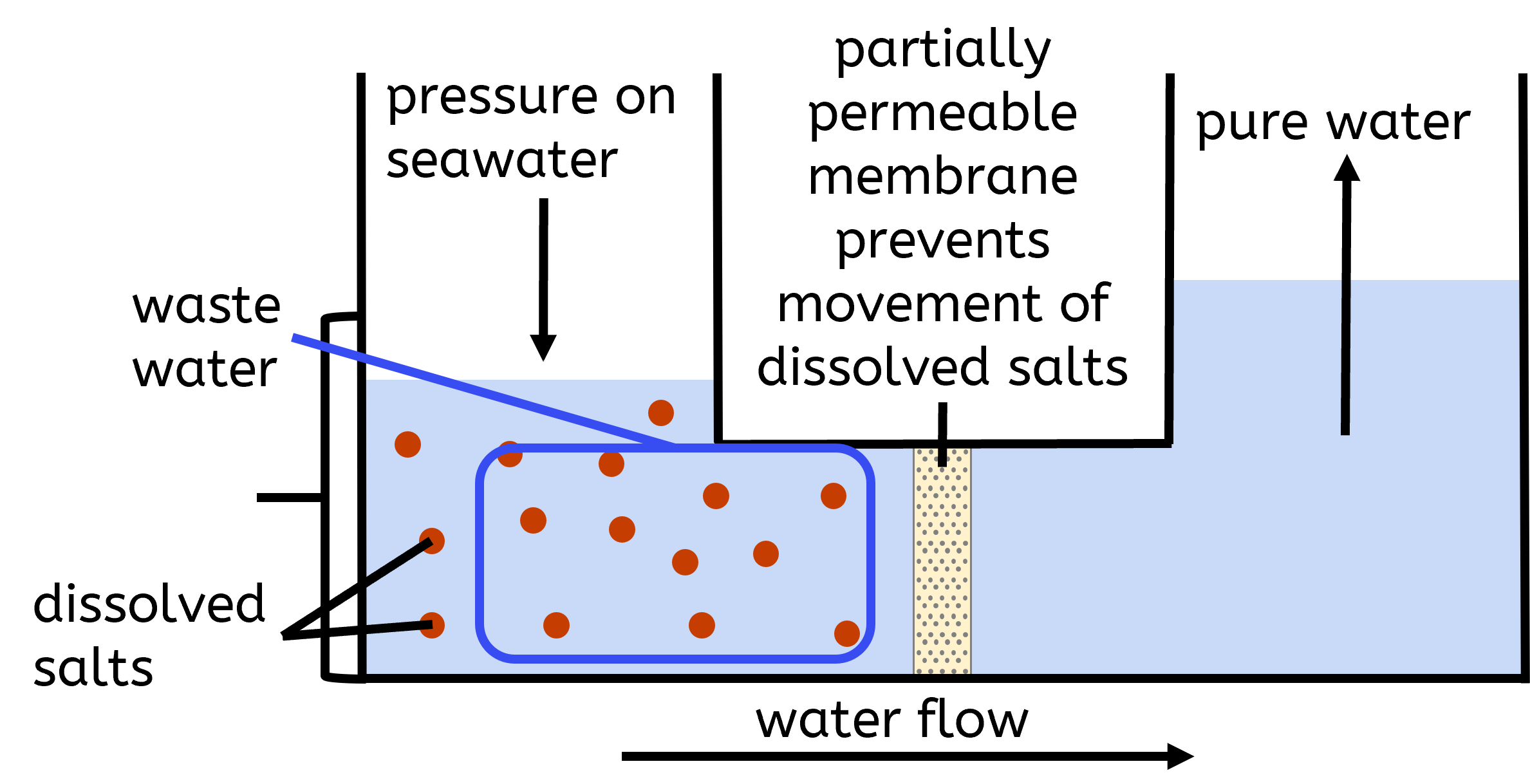
In regions where fresh water supplies are limited, desalination of salty water or seawater may be required. Desalination processes include distillation and reverse osmosis:
- Distillation involves heating the water until it evaporates, leaving the salts behind, and then condensing the vapour back into liquid.
- Reverse osmosis involves forcing water through a partially permeable membrane that blocks salts and other impurities while allowing water molecules to pass through.
Treating Different Types of Water
Both processes require significant amounts of energy, making them more expensive than traditional fresh water treatment methods.
Urban lifestyles and industrial processes produce large amounts of wastewater that require treatment before being released into the environment. Sewage and agricultural wastewater require the removal of organic matter and harmful microbes. Industrial wastewater may require the removal of organic matter and harmful chemicals. Sewage treatment includes several stages:
- Screening and Grit Removal: This step removes large debris and grit to protect the machinery used in the following stages.
- Sedimentation: The water is stored in large tanks where heavy particles settle to the bottom. This process produces sewage sludge (solid waste) and effluent (liquid waste).
- Aerobic biological treatment of effluent: The effluent is treated with bacteria that use oxygen to break down the remaining organic matter. This process cleans the water before it is released back into the environment.
- Anaerobic digestion of sewage sludge: In this stage, bacteria break down the organic matter in the sludge without using oxygen. This process reduces the volume of sludge and produces biogas, which can be used for energy.

Obtaining potable water from different sources involves varying levels of difficulty:
- Wastewater: Requires extensive treatment to remove organic matter, microbes, and harmful chemicals.
- Groundwater: Often needs less treatment as it is naturally filtered through soil and rocks.
- Saltwater: Desalination is required, which is energy-intensive and expensive.
Hard water
Water can be classified as hard or soft depending on its mineral content.
- Hard water: Contains high levels of dissolved minerals, primarily calcium and magnesium. These minerals come from rocks and soil that the water passes through before reaching our homes.
- Soft water: Contains low levels of dissolved minerals. Rainwater is naturally soft, but it can become hard as it flows over and through mineral-rich ground.
When hard water is used with soap, the calcium and magnesium ions react with the soap to form an insoluble substance called soap scum. This is why it is difficult to get a good lather with soap in hard water. Soap scum can leave a residue on skin, clothes, and surfaces, and it can be challenging to clean.
Hard water is not harmful to drink and may even have health benefits. The minerals in hard water can contribute to dietary calcium and magnesium intake. However, very hard water can sometimes be less palatable and may cause minor digestive issues in sensitive individuals. It can cause a build-up of limescale, a hard, chalky deposit that forms when the water is heated.

Limescale can accumulate in:
- Pipes: Reducing water flow and increasing the risk of blockages.
- Kettles and coffee makers: Reducing efficiency and lifespan.
- Washing machines and dishwashers: Leading to higher energy usage and potential damage to heating elements.
- Sinks, baths, and taps: Leaving unsightly deposits and reducing water flow.
Water softening is the process of removing calcium and magnesium ions from hard water. It prevents limescale build-up in pipes and appliances, which helps maintain efficient water flow and reduces the risk of blockages. Softened water also improves the effectiveness of soap and detergent, making cleaning tasks easier and more effective. Additionally, it reduces soap scum formation, leaving surfaces cleaner. By preventing limescale and soap scum, water softening prolongs the lifespan of appliances and plumbing systems, ensuring they operate more efficiently and last longer.
One of the most common softening method work as follows:
- Ion-exchange resin: The hard water passes through a resin bed containing sodium or potassium ions. The calcium and magnesium ions in the water are exchanged for sodium or potassium ions, which do not cause hardness.
Listen to this page (feature coming soon)
Did you know?
- The ancient Greeks and Romans used sand and gravel to filter their water, an early form of water treatment.
- Reverse osmosis was developed in the mid-20th century and is now widely used for desalination in many arid regions.
- Chlorine was first used as a water disinfectant in the early 1900s and has significantly reduced waterborne diseases.
Why do we care?
- Understanding the water cycle helps us manage water resources, predict weather patterns, and support agriculture by explaining rainfall and irrigation.
- Knowing how to make water potable ensures safe drinking water, preventing diseases in communities lacking clean water.
- Treating water, including sewage, is vital for maintaining public health and preventing environmental pollution, which can impact ecosystems.
- Understanding hard water helps prevent scale build-up in appliances like kettles and boilers, improving their efficiency and lifespan.
Key information
- Potable water is essential for life, containing low levels of dissolved salts and microbes, but it is not chemically pure.
- Pure water must be used in chemical analysis, to avoid unwanted impurities causing reactions (producing precipitates).
- The water cycle plays a crucial role in providing freshwater.
- The UK produces potable water through sedimentation, filtration, and sterilisation.
- Desalination is used when freshwater is scarce, and requires significant energy.