
States of Matter (GCSE)
KS4 National Curriculum Statement(s) covered
- Changes of state of matter in terms of particle kinetics, energy transfers
Skip to:
The three states of matter are solid, liquid, and gas. Each state is characterised by the arrangement and energy of its particles, which can be explained using particle theory.
Melting and freezing occur at the melting point, while boiling and condensing occur at the boiling point. The transition between these states involves energy transfers that depend on the strength of the forces between the particles of the substance.
Particles
In chemistry, the term "particles" refers to the small constituents that make up matter. These particles can be atoms, molecules, or ions, depending on the type of substance and its state of matter:
- Atoms are the basic building blocks of elements, consisting of protons, neutrons, and electrons.
- Molecules are small groups of atoms covalently bonded together.
- Ions are atoms or groups of atoms that have gained or lost electrons, carrying a charge.
In addition to these, there are even smaller particles such as subatomic particles (protons, neutrons, and electrons), as well as macromolecules and nanoparticles. Understanding these varying scales of particles helps explain a wide range of chemical phenomena and material properties.
States of Matter
The three main states of matter that we commonly interact with are solid, liquid, and gas. Each state is characterised by the arrangement and energy of its particles, which can be explained using particle theory.
| solid state | liquid state | gas state | |
|---|---|---|---|
| particle diagram | 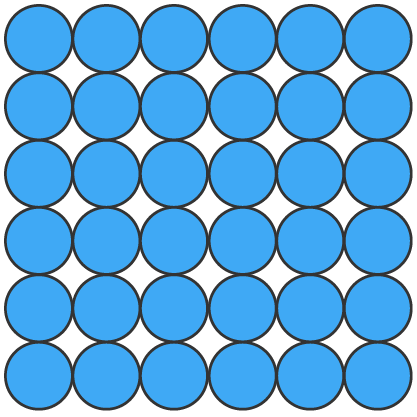 |
 |
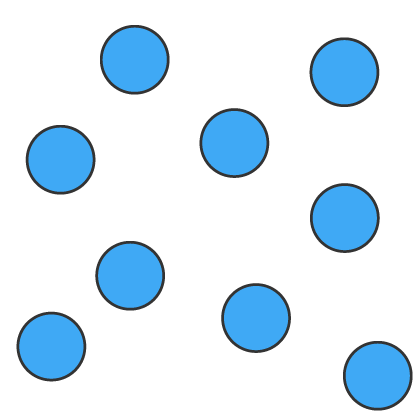 |
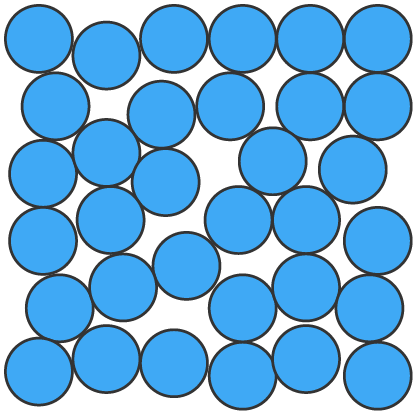
| arrangement | particles are closely packed in a fixed, orderly arrangement | particles are close together but not in a fixed position, allowing them to move past one another | particles are far apart and move freely and rapidly in all directions |
| particle energy | very little energy, particles only vibrate in place | moderate energy, particles move freely but stay close together | high energy, particles move freely and rapidly |
| shape of substance | definite shape | no definite shape, takes the shape of its container | no definite shape, expands to fill any container |
| volume of substance | definite volume | definite volume | no definite volume, expands to fill any container |
| 3D representation |  Animated Gif licensed under CC BY-SA 4.0 |
 Animated Gif licensed under CC BY-SA 4.0 |
 Animated Gif licensed under CC BY-SA 4.0 |
Substances in the gas state can be easily compressed because there are large spaces between particles. In contrast, substances in the solid and liquid state are not considered to be compressible due to the close packing of their particles.
Liquids and gases are considered fluids. Fluids are characterised by the ability of their particles to move past one another, allowing them flow and adapt to the shape of any container they are in.
While solid, liquid, and gas are the most commonly encountered states of matter, there are others as well. One notable example is plasma, which is a state of matter where the a gaseous substance is energised until atomic electrons are no longer associated with any particular atomic nucleus.
Plasmas are found naturally in stars, including the sun, and are used in fluorescent lights and plasma TVs. Other states of matter are typically encountered under extreme conditions, such as very high temperatures or pressures.
The states of matter of water (H₂O)
Water can exist in the main three states: solid, liquid, and gas. Each state has distinct properties and is named differently based on its state. It has the same chemical composition in all three states.
Solid state: ice
When water is in its solid state, it is called ice. Ice is rigid and maintains a fixed shape due to the structured arrangement of water molecules. This state occurs at temperatures below 0°C under normal atmospheric pressure.
Liquid state: (liquid) water
In its most familiar form, in its liquid state water is known as water (or liquid water). This state exists at temperatures between 0°C and 100°C under normal atmospheric pressure. Liquid water flows easily, takes the shape of its container, and has a fixed volume.
Gas state: water vapour (not necessarily steam)
When water is in its gaseous state, it is referred to as water vapour or steam. Water vapour is the true gaseous form of water, which is invisible and forms through evaporation at any temperature. Evaporation occurs when water molecules escape into the air from the surface of the liquid.
Steam is the term used for the visible mist that forms when water boils at 100°C under normal atmospheric pressure. As the water vapour cools, it condenses into tiny droplets, creating this mist. Although the term "steam" is often used to describe water in its gaseous state, it technically refers to the visible droplets formed from condensed water vapour.
State Changes
When a substance changes state, the energy and movement of its particles change.
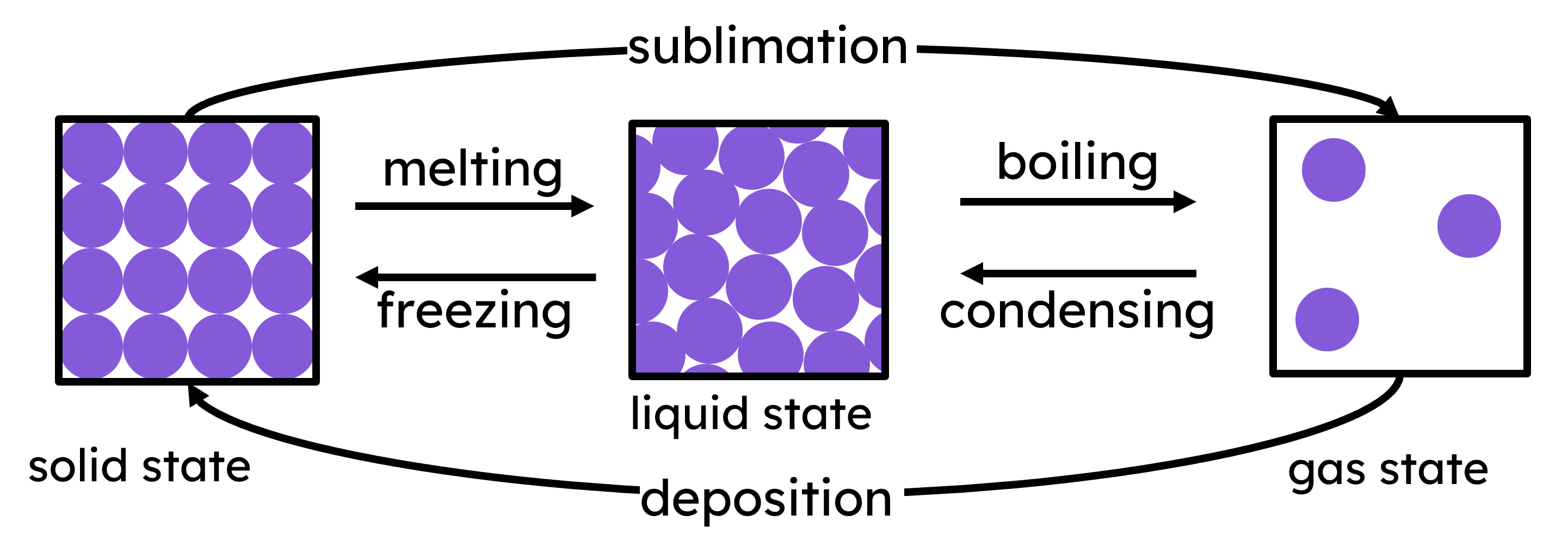
When a substance is heated, energy is absorbed from the surroundings and the movement of its particles increase, leading to state changes:
| state change | description |
|---|---|
| melting | a substance in the solid state absorbs enough energy for its particles to break free from their fixed positions, becoming a liquid |
| boiling | a substance in the liquid state gains enough energy for its particles to overcome the forces holding them in the liquid state and become a gas throughout the liquid |
| sublimation | a substance in the solid state changes directly into a gas without passing through the liquid state |
Boiling is a bulk process where a liquid changes to a gas at a specific temperature (the boiling point) and occurs throughout the liquid. Evaporation is a surface phenomenon that can happen at any temperature where only particles at the surface gain enough energy to become gaseous.
When a substance is cooled, energy is released to the surroundings and movement of its particles decrease, leading to state changes:
| state change | description |
|---|---|
| freezing | a substance in the liquid state loses energy and its particles slow down enough to form a solid structure |
| condensing | a substance in the gas state loses energy and its particles come together for form a liquid |
| deposition | a substance in the gas state changes directly into a solid without passing through the liquid state |
The term "freezing" is commonly used to describe the state change from liquid to solid. However, "solidifying" is a more general term that can be used to describe this process, especially in contexts where the temperature is not below the freezing point of water.
Heating curves
A heating curve is a useful way to illustrate how the temperature of a substance changes as it is heated at a constant rate. When a pure substance undergoes a state change, such as melting or boiling, the change occurs at a specific temperature, even if heating continues. This is because the added energy is used to overcome forces of attraction between particles rather than increase the temperature.
The graph below shows the temperature of a substance measured every minute as it is heated at a constant rate, undergoing one change of state. The shape of the graph can be divided into three sections, each representing different stages of heating and phase change.
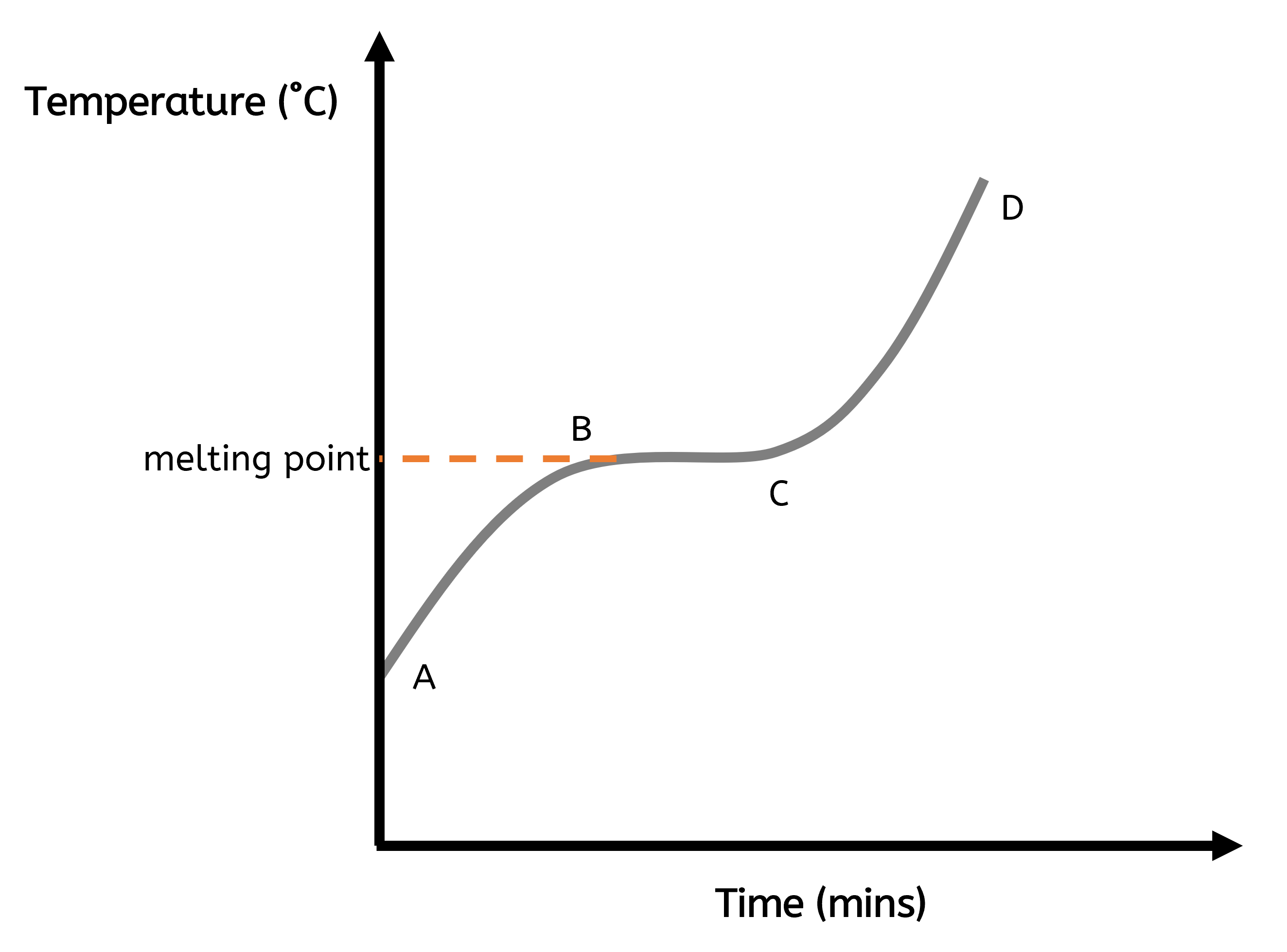
Section A to B: Heating the Solid
In the initial stage, from point A to point B, the graph shows a steady rise in temperature. During this phase, the substance is in a solid state. The particles are arranged in a fixed lattice structure and occupy fixed positions. As heat is applied, the energy from the heat source is transferred to the particles. This causes the particles to vibrate more rapidly. The increase in particle vibrations raises the temperature of the solid until it reaches its melting point.
Section B to C: Melting
From point B to point C, the graph becomes flat, indicating that the temperature remains constant despite continuous heating. This flat section corresponds to the melting process, where the solid is changing into a liquid. During melting, the energy supplied is used to break the forces of attraction holding the particles in the solid lattice. This energy is stored in the particles as potential energy, allowing them to move out of their fixed positions and transition into a liquid state. The temperature does not increase during this phase because all the added energy is used for the state change rather than increasing the thermal energy store of the particles.
Section C to D: Heating the Liquid
In the final stage, from point C to point D, the graph shows a steady increase in temperature again. At this point, the substance is completely in the liquid state. The particles in the liquid move past one another more freely and randomly. As heating continues, the energy from the heat source continues to be transferred to the particles, increasing their thermal energy store and causing the temperature of the liquid to rise and the particles to move more rapidly.
Melting and Boiling Points
The melting point and boiling point are characteristic physical properties of a substance, used to identify and classify materials:
- Substances with high melting and boiling points, like metals, require a lot of energy to transition to the liquid or gas state, making them suitable for high-temperature applications.
- Conversely, substances with low melting and boiling points can be useful in situations where easy melting or boiling is desired, such as in certain manufacturing processes.
The melting point of a substance is the same as its freezing point. This is the temperature at which a substance can exist in both solid and liquid states, depending on whether it is absorbing or releasing energy. At this specific temperature, the particles in the substance have enough energy to overcome the forces holding them in the solid state and start moving freely, forming a liquid.
Conversely, when a substance in the liquid state cools down to this temperature, the particles lose energy, slow down, and begin to arrange themselves into a fixed, orderly pattern, forming a solid.
Similarly, the boiling point of a substance is the same as its condensing point. This is the temperature at which a substance can exist in both liquid and gas states. When a substance in the liquid state reaches its boiling point, it absorbs enough energy for its particles to overcome any forces of attraction and become gaseous. Conversely, at the condensing point, particles lose energy and come together to form a liquid.
The boiling point of a substance is the temperature at which its vapour pressure equals the external pressure. At lower air pressure, the vapour pressure required for a liquid to boil is achieved at a lower temperature. Therefore, on a mountain, water boils at a lower temperature than 100°C. For example, on Mount Everest, water boils at around 68°C. This means that food cooked by boiling may take longer to cook because the temperature is not as high.
Understanding how to predict the state of a substance at different temperatures is a crucial skill in chemistry. By using a number line, you can visualise the temperature ranges for different states of matter and determine whether a substance will be a solid, liquid, or gas at a given temperature:
- Identify the melting and boiling points:
- To predict the state of a substance, you first need to know its melting and boiling points.
- These temperatures mark the transitions between solid, liquid, and gas states.
- Create a number line:
- Draw a horizontal number line and mark the melting point and boiling point of the substance on it.
- Label the regions between these points to indicate the different states.

- Determine the state:
- Using the number line, you can easily determine the state of the substance at any given temperature by seeing where the temperature falls in relation to the melting and boiling points.
Worked Example - water (H₂O)
What is the physical state of water at 20°C?
- Melting Point: 0°C
- Boiling Point: 100°C
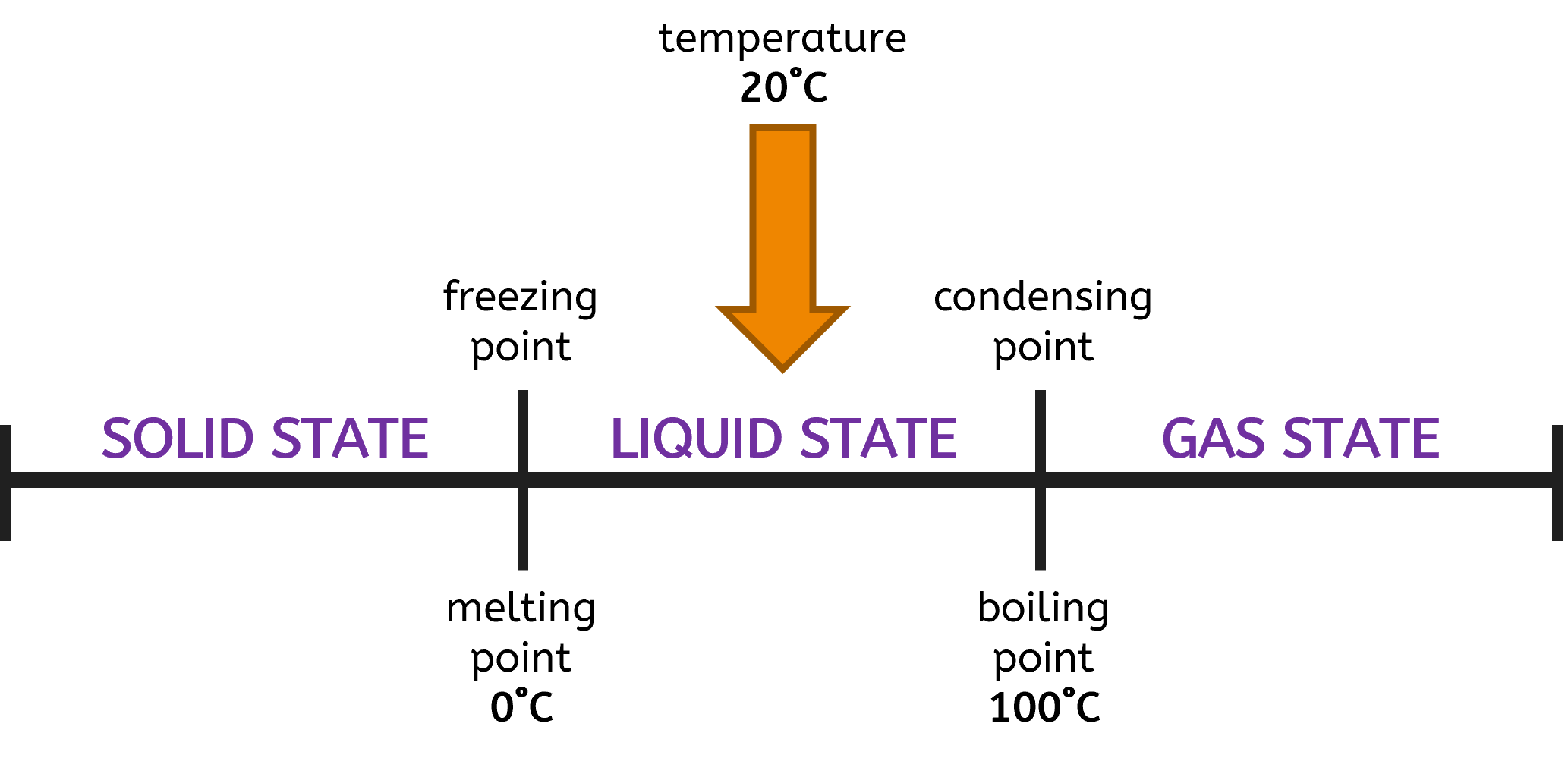
Using the number line, you can easily determine the state of the substance at any given temperature by seeing where the temperature falls in relation to the melting and boiling points.
- Below 0°C: Water is in the solid state (ice).
- At 0°C: Water can exist as both solid and liquid (melting or freezing).
- Between 0°C and 100°C: Water is in the liquid state.
- At 100°C: Water can exist as both liquid and gas (boiling or condensing).
- Above 100°C: Water is in the gas state (steam).
Worked Example - ethanol (C₂H₆O)
What is the physical state of ethanol at -120°C?
- Melting Point: -114°C
- Boiling Point: 78°C
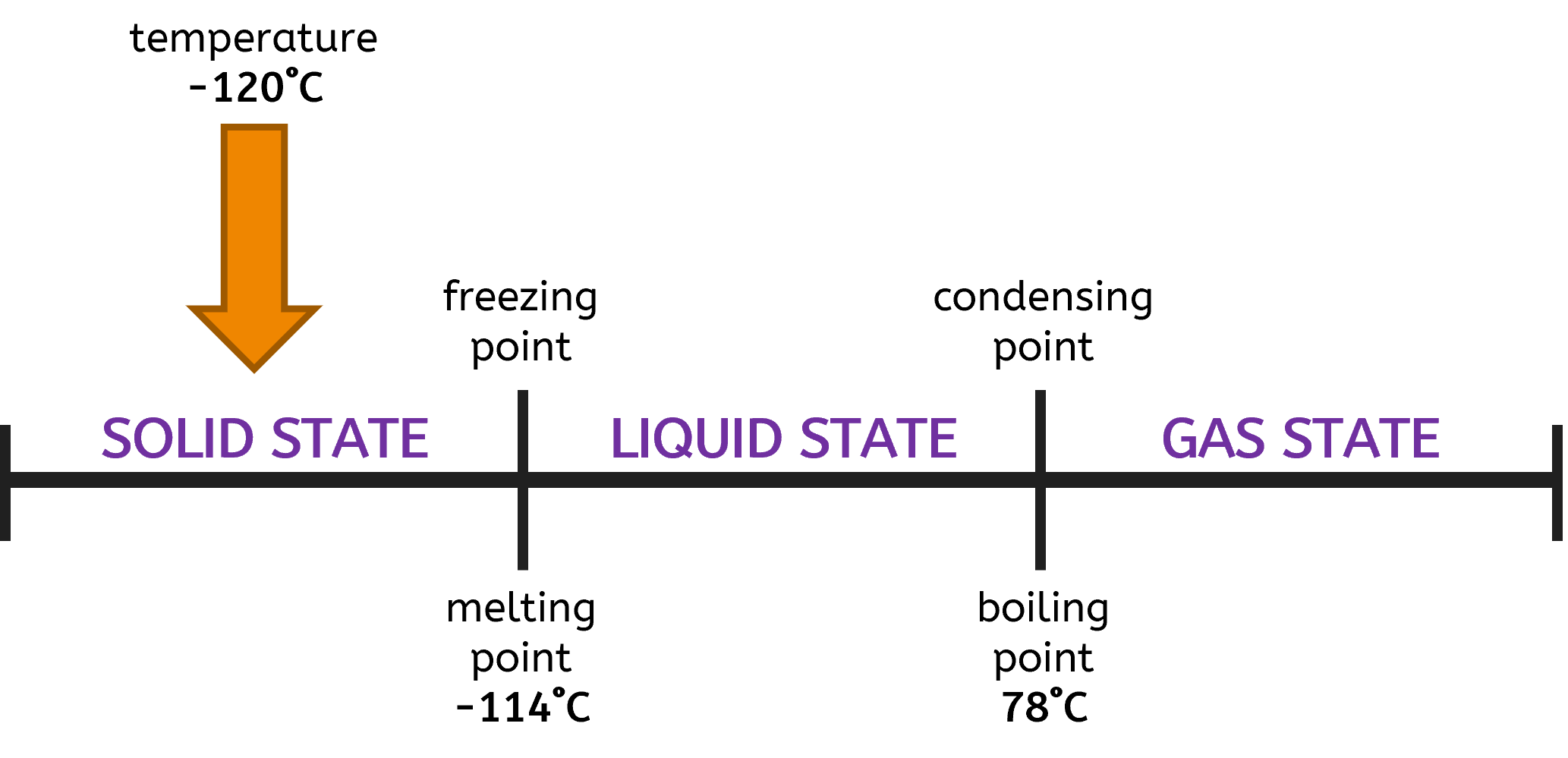
Using the number line, you can easily determine the state of the substance at any given temperature by seeing where the temperature falls in relation to the melting and boiling points.
- Below -114°C: Ethanol is in the solid state.
- At -114°C: Ethanol can exist as both solid and liquid.
- Between -114°C and 78°C: Ethanol is in the liquid state.
- At 78°C: Ethanol can exist as both liquid and gas.
- Above 78°C: Ethanol is in the gas state.
Limitations of the Particle Model
The simple particle model represents particles as small solid spheres without forces between them.
However, this model has limitations:
- It does not account for the forces of attraction between particles, which influence the energy needed for state changes.
- Real particles are not perfect spheres and are not solid; they have internal structures and can be influenced by various forces.
- This model also does not adequately explain the energy changes and movement during state transitions.
Listen to this page (feature coming soon)
Did you know?
- Under certain conditions, water can be cooled below its freezing point without forming ice, a process known as supercooling. If the supercooled water is disturbed, it can instantly freeze.
- The concept of melting points is crucial in cooking. For example, chocolate must be tempered by carefully melting and cooling it to achieve the right texture and shine.
- Water has more than 20 different crystalline states of ice, known as ice polymorphs. These forms of ice occur under various temperature and pressure conditions, each with unique molecular arrangements.
Why do we care?
- Knowing about state changes and melting/boiling points is important for everyday activities like boiling water for cooking and understanding why ice cream melts on a hot day.
- Realising the limitations of the particle model helps us improve scientific models and predictions, crucial for advanced studies and practical applications like developing new materials.
Key information
- Solids, liquids, and gases are characterised by particle arrangement and energy.
- Heating increases energy and particle movement; cooling decreases them.
- Melting/Freezing Point: This is the same temperature where a substance can exist as both solid and liquid.
- Gases are easily compressed due to spaces between particles; solids and liquids are considered to be not compressible.
- Boiling occurs throughout the liquid at a specific temperature; evaporation happens at the surface and can occur at any temperature.
- The simple particle model doesn't account for forces between particles and oversimplifies their structure.